Process for producing L-lysine by fermenting
A lysine, amino acid technology, applied in the field of DNA and microorganisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
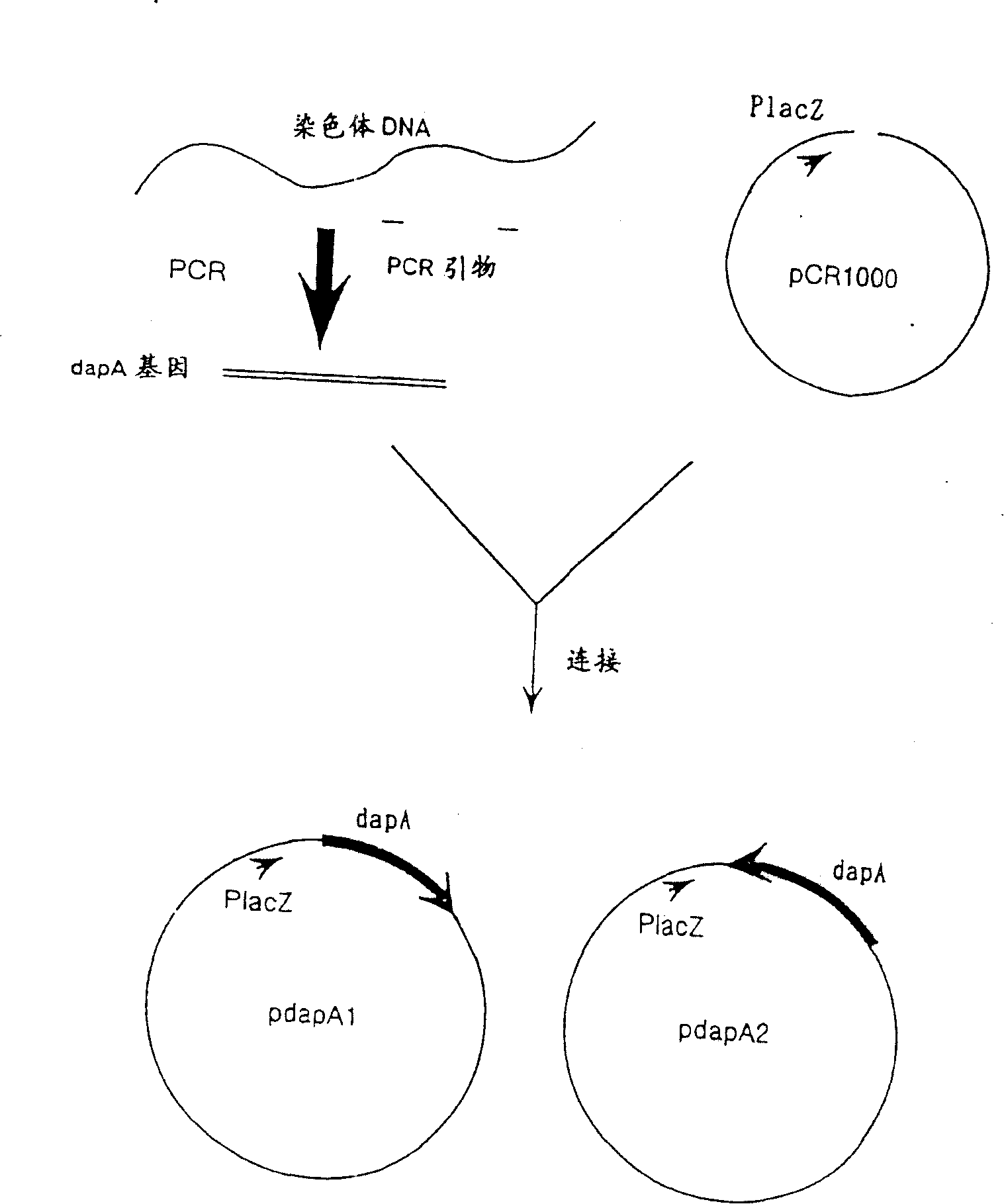
[0155] Embodiment 1: Preparation of mutant DDPS gene
[0156] (1) Cloning of wild-type dapA gene
[0157] The nucleotide sequence of the dapA gene of E. coli has been reported (Richaud, F. et al., J. Bacteriol., 297 (1986)), and its known openable frame (ORF) includes 876 base pairs and Encodes a protein comprising 292 amino acid residues. Since it is unknown how the dapA gene is regulated, only the SD sequence and the ORF region except the promoter region were amplified and cloned by PCR method.
[0158] The total genomic DNA of E. coli K-12MC1061 strain was extracted by the same method as that of Saito and Miura (Biochem. Biophys. Acta., 72, 619 (1963)). Prepare two kinds of primers with sequences shown in SEQ ID NO: 1 and NO: 2, and use the primers to complete the PCR reaction and amplify the target DNA according to the method consistent with Erlich et al. (PCR Technology, Stockton press (1989)) . The obtained DNA was inserted into a commercially available cloning vec...
Embodiment 2
[0202] Embodiment 2: Preparation of mutant AKIII gene
[0203] (1) Cloning of wild-type lysC gene
[0204] The nucleotide sequence of the E. coli AKIII gene (lysC) has been reported (Cassan, M., Parsot, C., Cohen, G.N., and Patte, J.C., J. Biol. Chem., 261, 1052 (1986)) , and its known translational frame (ORF) comprises 1347 base pairs, and encodes a protein comprising 449 amino acid residues. In this gene, there is an operon, which is repressed by L-lysine. Therefore, in order to remove this operon region, the region containing only one SD sequence and ORF was amplified and cloned by PCR.
[0205] Total genomic DNA of E. coli K-12MC1061 strain was prepared according to the method of Saito and Miura (Biochem. Biophys. Acta., 72619 (1963)). Two primers having the sequences shown in SEQ ID NO: 5 and SEQ ID NO: 6 were prepared, and a PCR reaction was performed according to the method of Erlich et al. (PCR Technology, Stocktonpress (1989)) to amplify the lysC gene. The obta...
Embodiment 3
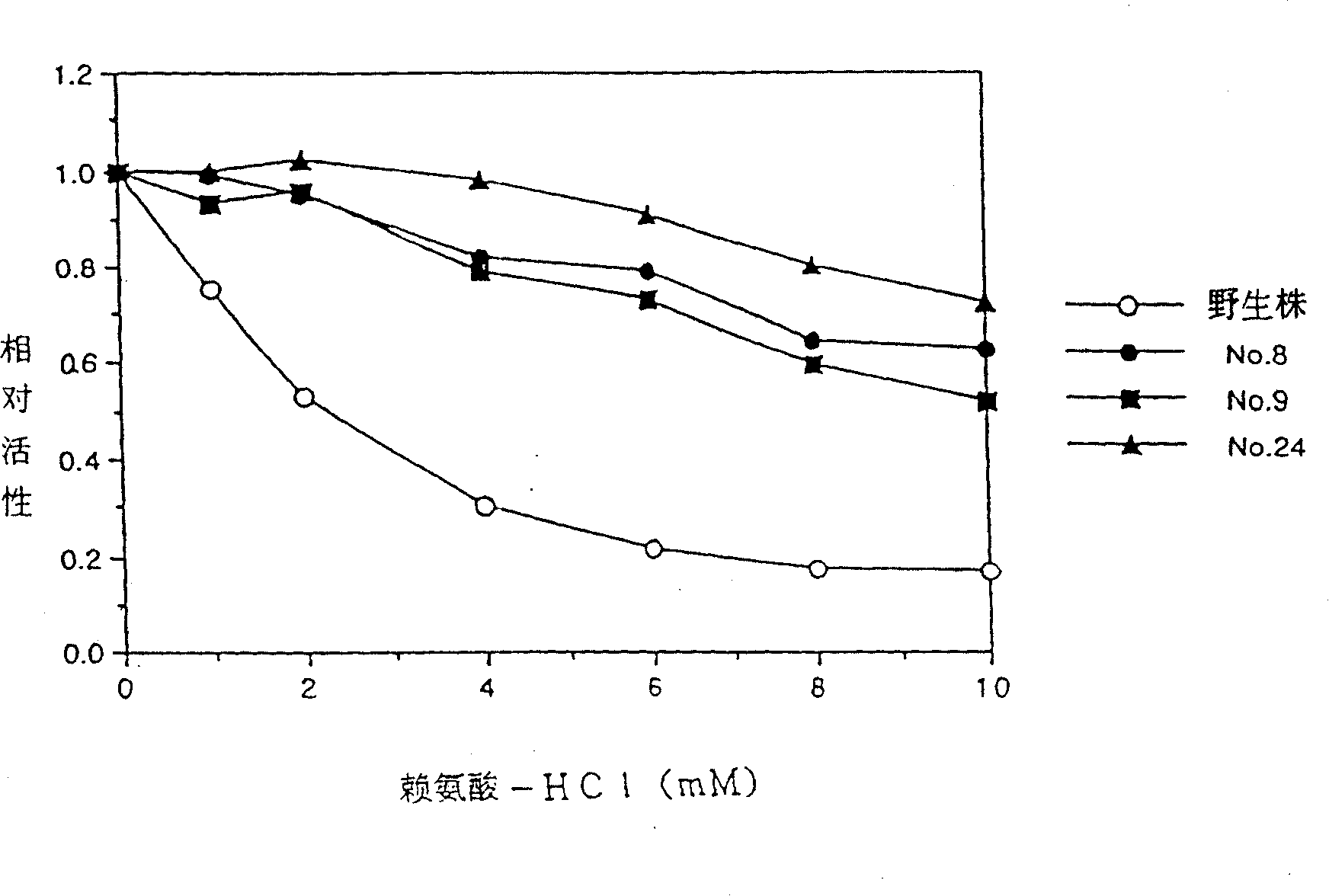
[0263] Example 3: import dapA with * Strains fermented to produce L-lysine
[0264] In order to produce L-lysine with E. coli, as pointed out in Japanese Patent Application Laid-open No. 56-18596, U.S. Patent No. 4,346, 170 and Applied Microbiology and Biotechnology, 15, 227-231 (1982), for Enhancing DDPS hosts is required for aspartokinase, which has been altered not to be inhibited by L-lysine. L-threonine-producing bacteria can be exemplified as such strains. Because of E. coli producing L-threonine, B-3996 has the highest yield among currently known strains. So the B-3996 strain was used to determine dapA * the host. The B-3996 strain extrachromosomally anchored pVIC40 as the only plasmid. See Japanese Patent Laid-open No. 3-501682 (PCT) for details. This microorganism is deposited with the Research Institute for Genetics and Industrial Microorganism Breeding under accession number RIA1867.
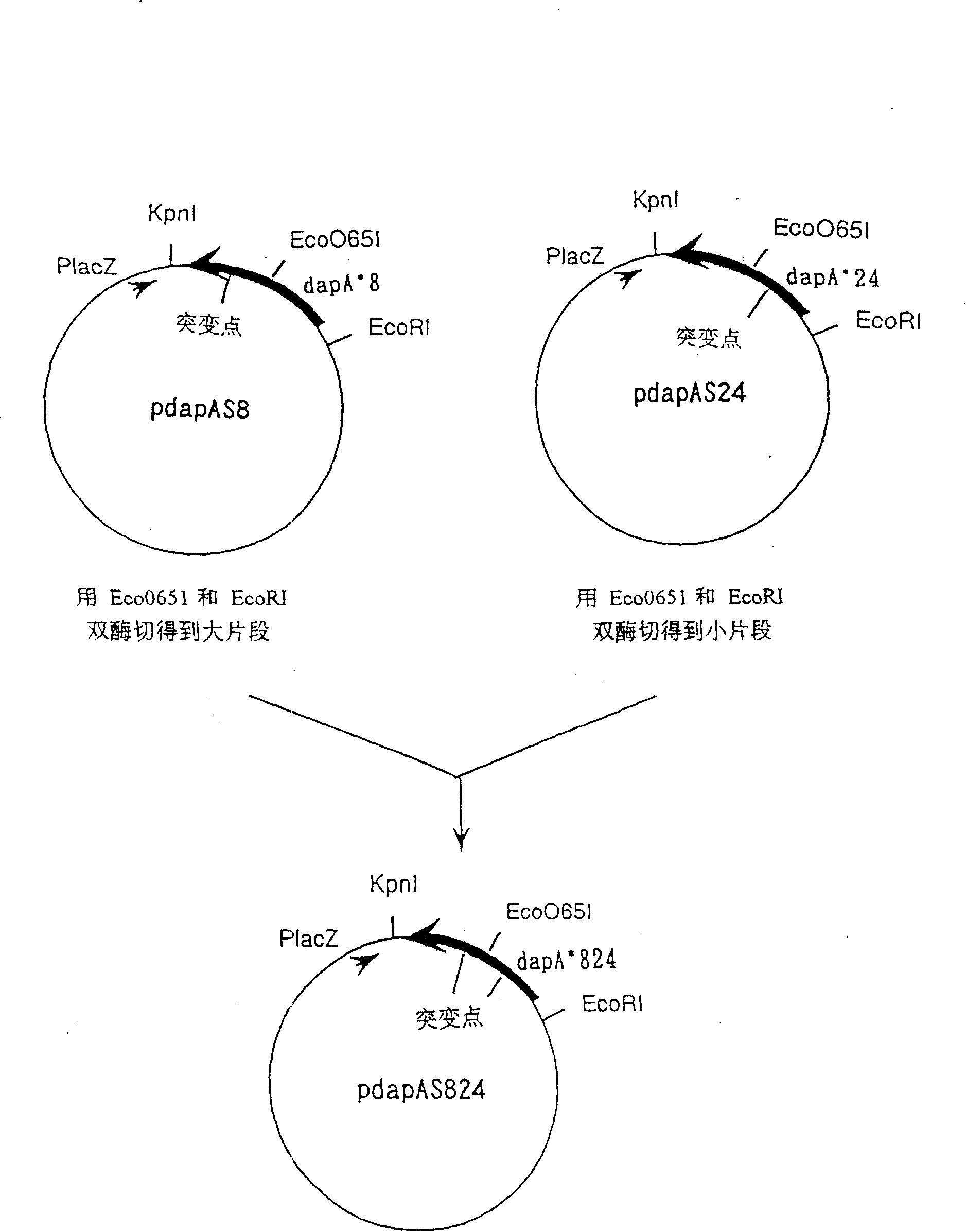
[0265] On the other hand, dapA present in pdapAS24 (in which the 118th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com