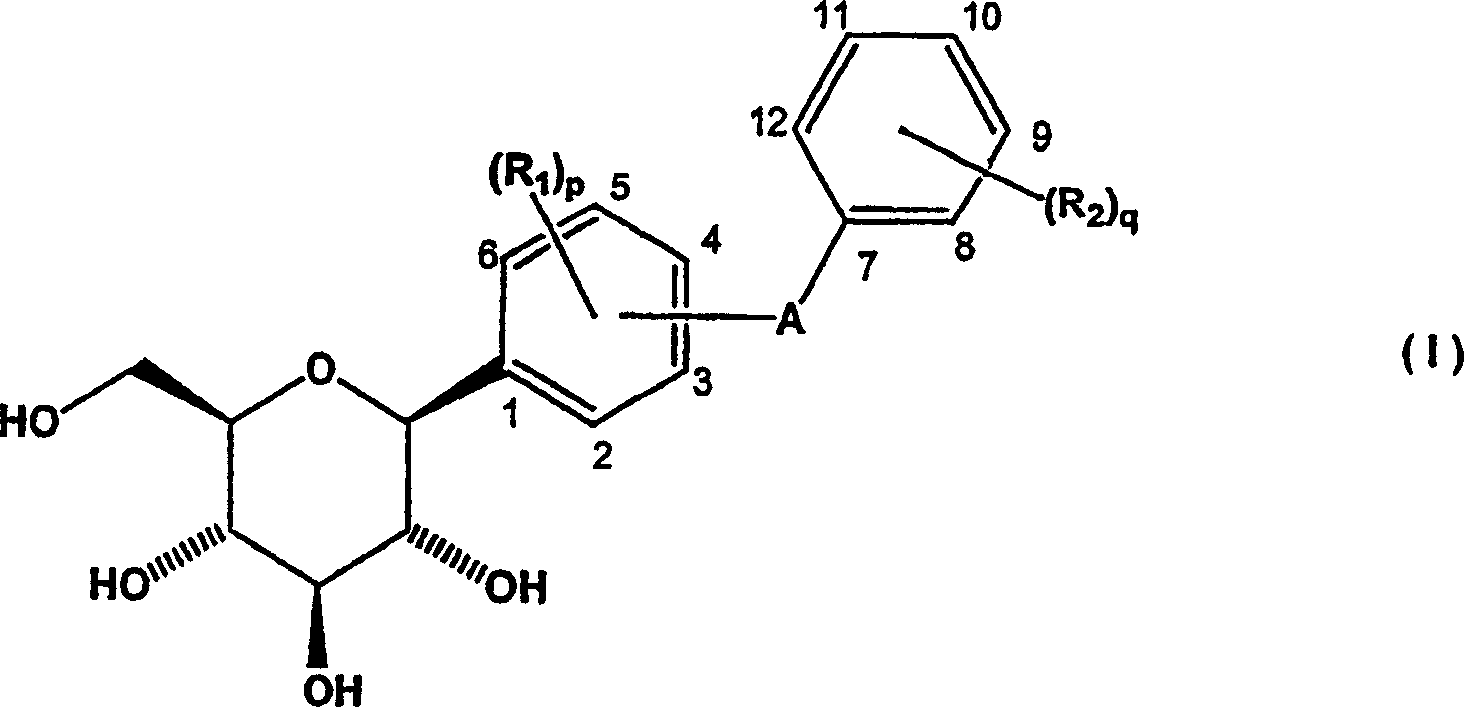
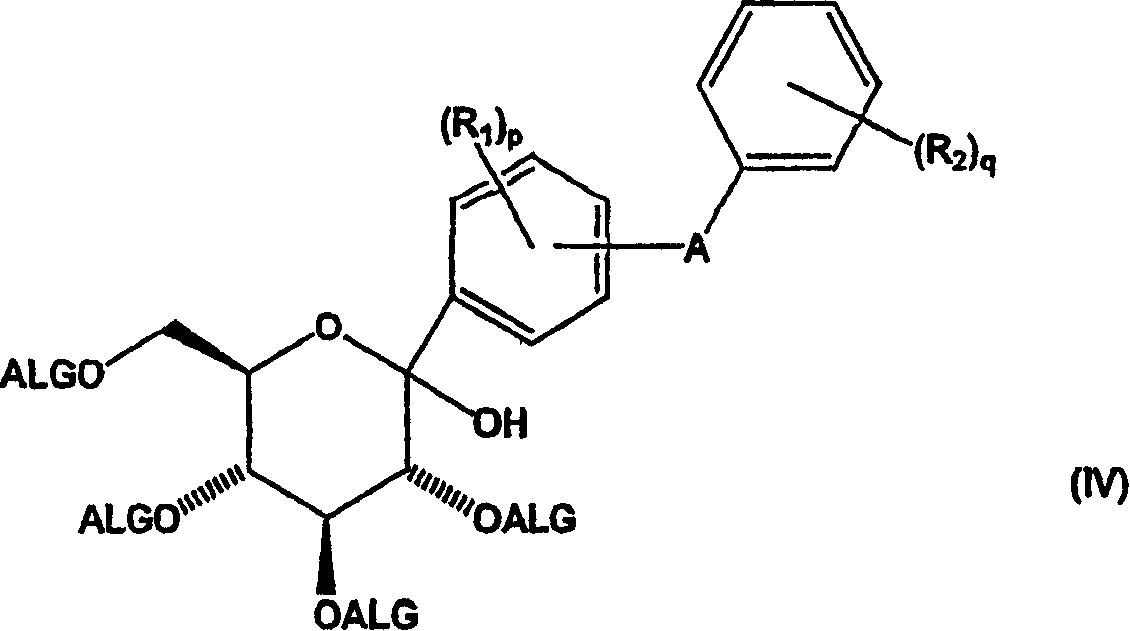
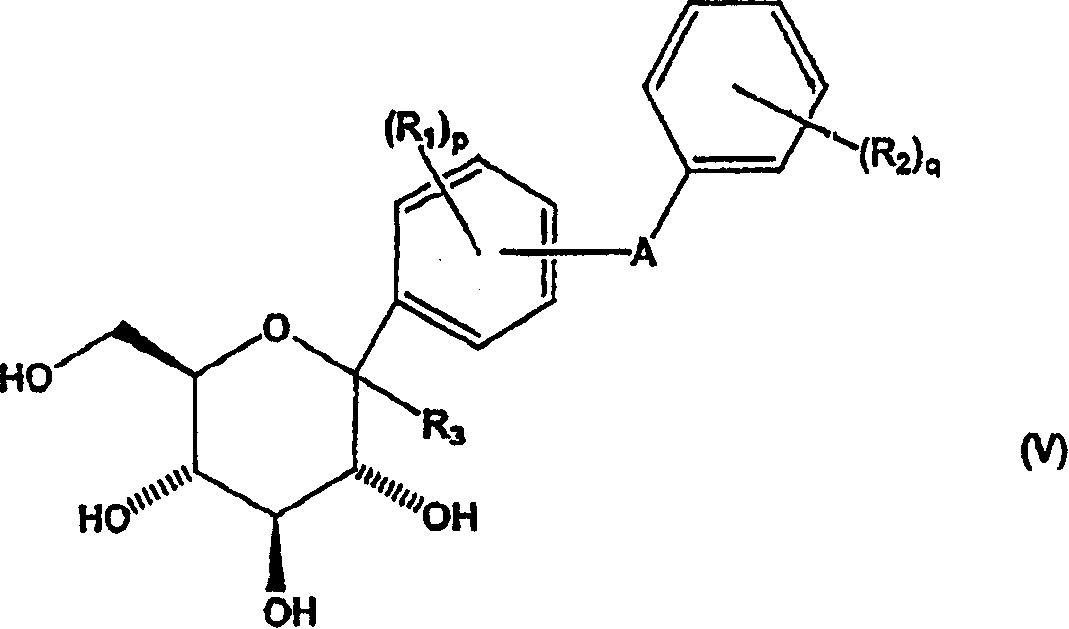
Methods of producing C-aryl glucoside SGLT2 inhibitors
An arylthio, CH2 technology, applied in the field of preparing C-aryl glucoside SGLT2 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0209] Preparation of 2,3,4,6-tetra-O-trimethylsilyl-1-C-(6-methyl-4′-(methylthio)diphenyl Methane-3-yl)-α-D-glucopyranose
[0210]
[0211] In a 1 L single-necked round bottom flask, aryl bromide compound (1) (20.7 g, 67.4 mmol, 1.1 eq) was dissolved in tetrahydrofuran (THF) (61 mL) and heptane (245 mL), cooled to -78 °C, Precipitation occurs. To the heterogeneous reaction mixture was added dropwise 2.3M n-BuLi (29.3 mL, 67.4 mmol) over 20 minutes, resulting in a reddish color. After 30 minutes, the reaction mixture was transferred into a 2-L single-necked flask containing trimethylsilyl lactone compound (2) (29.5 g, 63.2 mmol, 1 equiv) and heptane (306 mL) at -78 °C , a cloudy mixture was obtained without any precipitate. The reaction mixture was removed from the cold bath, quenched with 1% AcOH (290 mL), and transferred to a separatory funnel. 200 mL ethyl acetate (EtOAc) was added and the layers were separated. The organic layer was washed with water (1 x 200 mL) ...
Embodiment 2
[0213] Preparation of methyl-1-C-(6-methyl-4′-(methylthio)diphenylmethane-3-yl)-α-D-glucopyranose glucose
[0214]
[0215] The TMS-protected compound (3) (48 g) of Example 1 was dissolved in MeOH (196 mL), then methanesulfonic acid (200 μL) was added. The resulting solution was warmed to 40°C for about 20 minutes. The solution was then cooled to room temperature and concentrated. The residue was dissolved in EtOAc (200 mL), washed with saturated NaHCO 3 Aqueous solution (2 x 100 mL) and brine (2 x 100 mL) washed. The combined aqueous layers were back extracted with EtOAc (2×100 mL), and the combined organic layers were washed with Na 2 SO 4 Dry, filter and concentrate. The residue was dried under high vacuum overnight, then dissolved in toluene (ca. 75 mL) at 60 °C. The resulting mixture was added dropwise to a round bottom flask containing 450 mL of heptane, resulting in a white precipitate. The mixture was stirred at room temperature for about 3 hours and then...
Embodiment 3
[0217] Preparation of 1-C-(6-methyl-4′-(methylthio)diphenylmethane-3-yl)-β-D-glucopyranose
[0218]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com