Variable region of light chain and heavy chain genes of anti-KDR monoclonal antibody and its use
A variable region and antibody technology, applied in the application field of diagnosis and therapeutic drugs, can solve the problems of limited application and achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1, Cloning of Anti-KDR Antibody Light and Heavy Chain Variable Region Genes
[0021] 1. Extraction of total RNA: extract total RNA,
[0022] 2. Reverse transcription: Take 2μg total RNA and place it in a 0.5ml Eppendorf tube, add 5×RTbuffer, 0.1M DTT, 100ng random primers, 10nmol each of 4×dNTP, Rnasin 20U, put in a water bath at 65°C for 5-10min, then add 200U of M-MLV reverse transcriptase, placed in a 37°C water bath for 1 hour, then transferred to a 72°C water bath for 10 minutes.
[0023] 3. PCR amplification of the light and heavy chain variable region genes: Take 8 μl of the reverse transcription product and put it in a PCR tube, add 2 μl of 10mM dNTP, 10 μl of 10×PCR buffer, 2U of Pyrobest DNA polymerase, and use universal degenerate primers P1 and P2 respectively , 30pmol each of P3 and P4 specifically amplified the heavy chain and light chain variable region genes of the antibody, and introduced MuI I and Not I restriction endonuclease sites at the 5...
Embodiment 2
[0030] Example 2, Construction of Single Chain Antibody Gene Expression Vector pAYZKDR ScFv
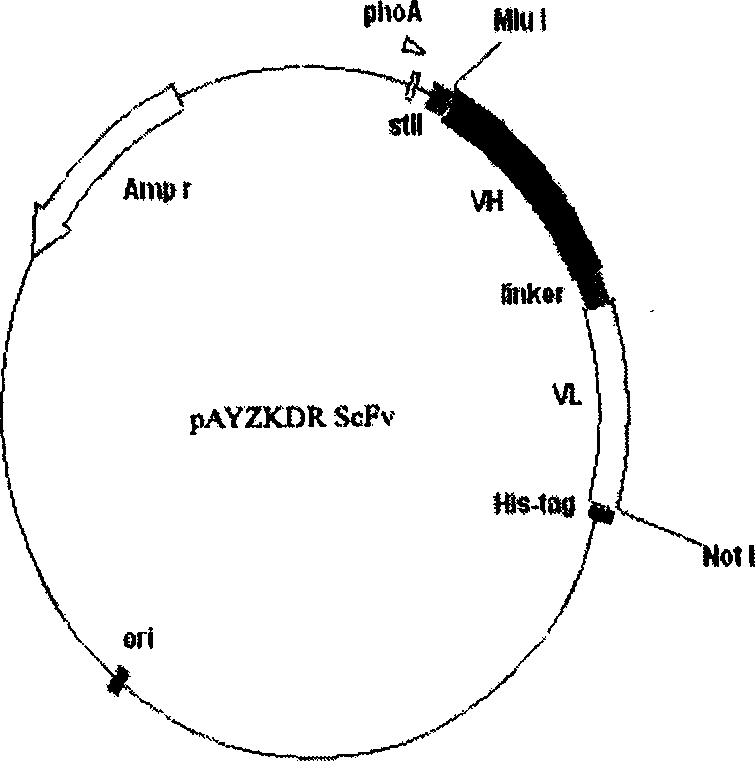
[0031] According to the enzyme cleavage map of the light and heavy chain variable regions and the enzyme cleavage site of the construction vector pAYZ, designed and synthesized for V H , V L Primers for gene amplification and splicing. First, primers P5, P6, P7, and P8 were used to amplify the heavy chain and light chain variable region genes respectively, and then the antibody light and heavy chain variable region genes were amplified with a DNA fragment encoding (G4S)3 short peptide by the overlap-PCR method. spliced into 5′V H -Linker-V L 3' fragment, and finally use P5 and P8 to amplify the full-length ScFv gene, and introduce Mull I and Not I restriction endonuclease sites at the 5' end and 3' end respectively. The gene was loaded into the pAYZ expression vector, constructed as figure 1 The indicated anti-KDR ScFv expression vector pAYZKDR ScFv.
[0032] The above three P...
Embodiment 3
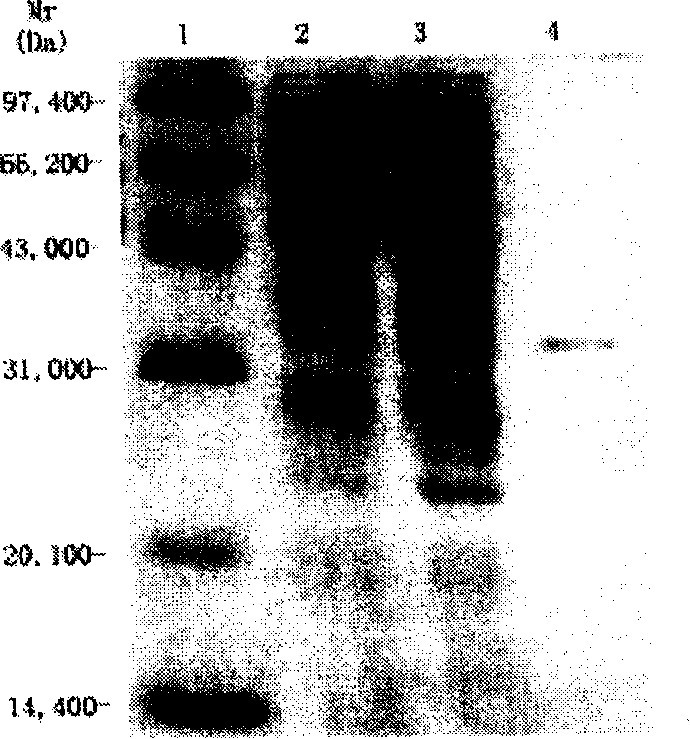
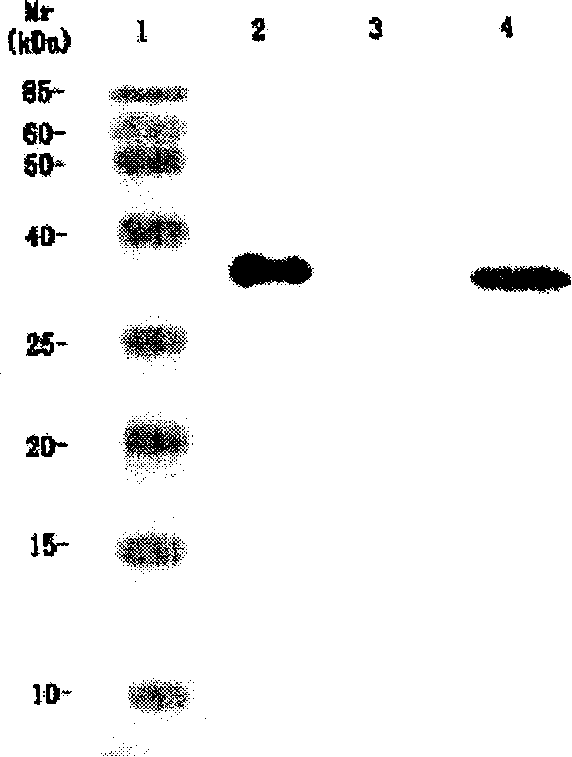
[0038] Example 3. Expression, purification and renaturation of anti-KDR ScFv antibody fragments
[0039] 1. Expression of anti-KDR ScFv antibody fragments
[0040] Transform E.Coli 16C9 with the constructed pAYZKDR ScFv plasmid, pick a single colony and inoculate it in 5ml of 2×YT medium containing 100mg / L ampicillin (each 1000ml contains 16g of peptone, 10g of yeast extract, 5g of sodium chloride, pH 7.4 ), 37°C, 250rpm, shaking culture for 10h; 4000rpm, 4°C centrifugation for 10min to collect the bacteria, resuspended in 20ml induction medium containing 100mg / L ampicillin (1.5g glucose, 11g tyrosine protein hydrolyzate per 1000ml , yeast extract 0.6g magnesium sulfate 0.19g, ammonium chloride 1.07, potassium chloride 3.73g, sodium chloride 1.2g, 1mol / L triethanolamine 120ml, pH7.4), 30°C, 250rpm, shaking culture for 24h; The cells were collected by centrifugation at 8000 rpm and 4°C for 10 min.
[0041] The cell pellet was resuspended in 1 / 30 culture volume of ice-precoole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com