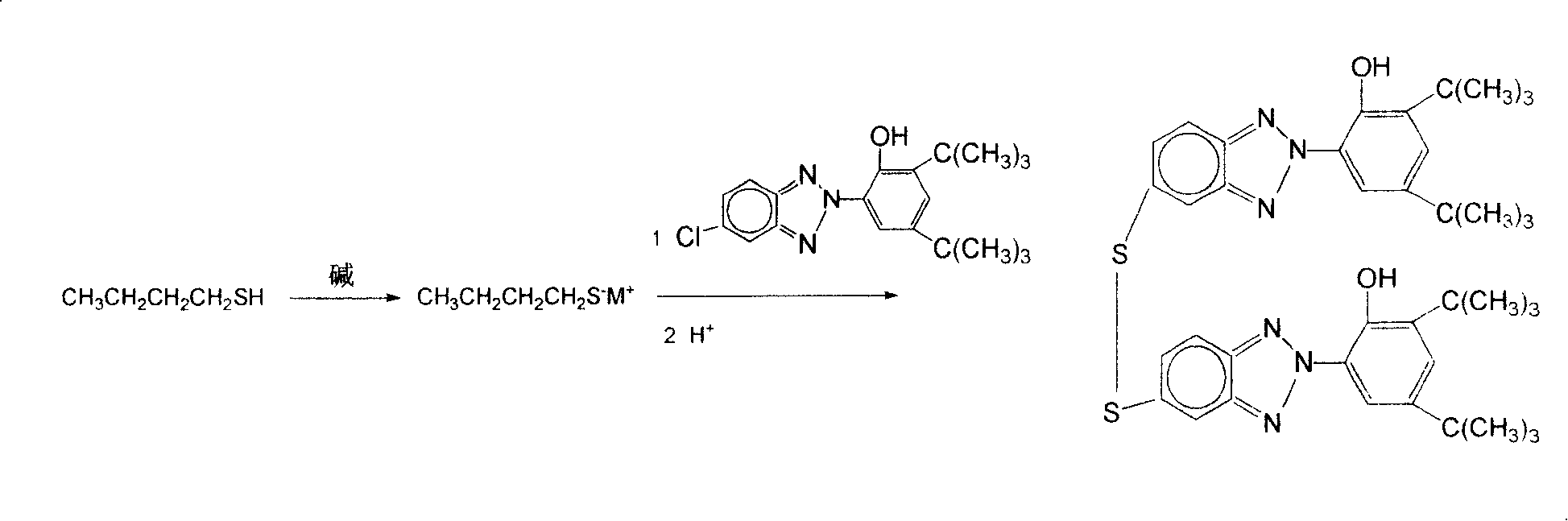
Benzotriazole compound containing sulfur, and its preparing method
A benzotriazole and benzotriazole technology are applied in the field of sulfur-containing benzotriazole compounds and their preparation, can solve problems such as unreported, and achieve the effect that the preparation method is simple and feasible
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Put a 250ml four-neck flask on a magnetic stirrer with a heating device, connect a nitrogen inlet tube, install a thermometer and a water separator condenser tube, add 3.2 grams of sodium hydroxide (0.08mol) and 7.25 grams of n-butylsulfide Alcohol (0.08 mol). Stirring was started and the sodium hydroxide was dissolved by slight heating. A further 12.5 ml of toluene was added, the mixture was heated to reflux to drive off the water formed (approximately 1.4 ml), and the toluene was distilled off.
[0029] Then the reaction mixture was cooled to room temperature, the water separator was removed, and the condenser was directly connected to the grinding port of the flask. Then add 30ml of N-methylpyrrolidone (0.31mol), 7.16 grams of 2-(2'-hydroxyl-3', 5'-di-tert-butylphenyl)-5-chlorobenzotriazole (0.02 mol), heated to 186°C, and reacted for 18 hours. The solution was cooled to room temperature. Slowly add 7.5ml of water and 7.5ml of concentrated hydrochloric acid under...
Embodiment 2
[0036] Put a 250ml four-neck flask on a magnetic stirrer with a heating device, connect a nitrogen inlet tube, install a thermometer and a condenser tube, add 7.25 grams of n-butanethiol (0.08mol), and add 2.3 grams of sodium hydride under stirring (0.096 mol).
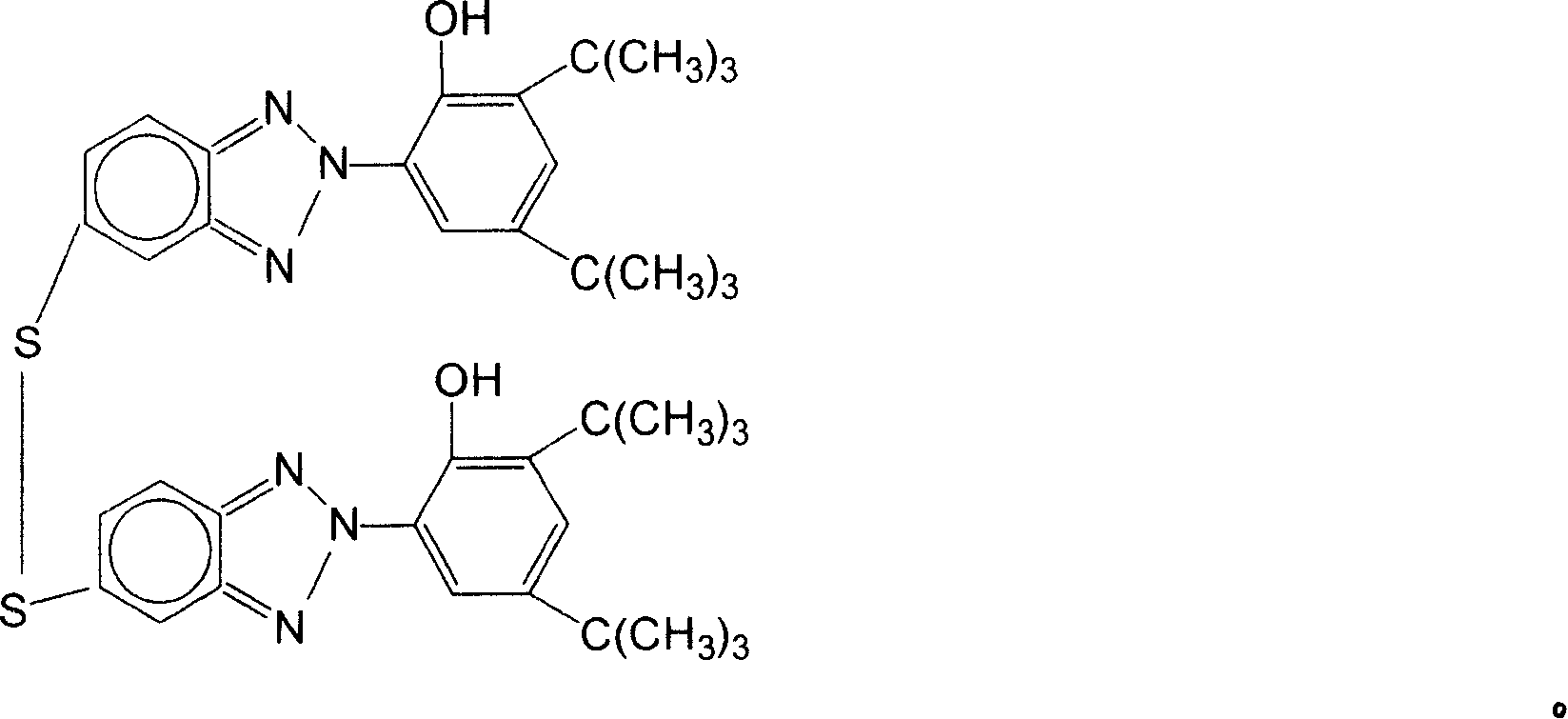
[0037] Add 30ml N-methylpyrrolidone (0.31mol) and 7.16 grams of 2-(2'-hydroxyl-3', 5'-di-tert-butylphenyl)-5-chlorobenzotriazole ( 0.02mol), heated to 186°C, and reacted for 18 hours. The solution was cooled to room temperature. Slowly add 9.3ml of water and 9.3ml of concentrated hydrochloric acid under stirring, a precipitate precipitates out after cooling, filter, wash the precipitate with petroleum ether, and dry to obtain 5,5'-dithio-bis[2-(2-hydroxy-3, 5-di-tert-butylphenyl)-2H-benzotriazole], the yield was 60%.
[0038] Physicochemical properties, spectral data and UV absorption of 5,5'-dithio-bis[2-(2-hydroxy-3,5-di-tert-butylphenyl)-2H-benzotriazole] prepared in this example (measure in chloroform) charact...
Embodiment 3
[0040] Put a 250ml four-neck flask on a magnetic stirrer with a heating device, connect a nitrogen inlet tube, install a thermometer and a condenser tube, add 7.25 grams of n-butanethiol (0.08mol), and add 1.92 grams of sodium hydride under stirring (0.08 mol).
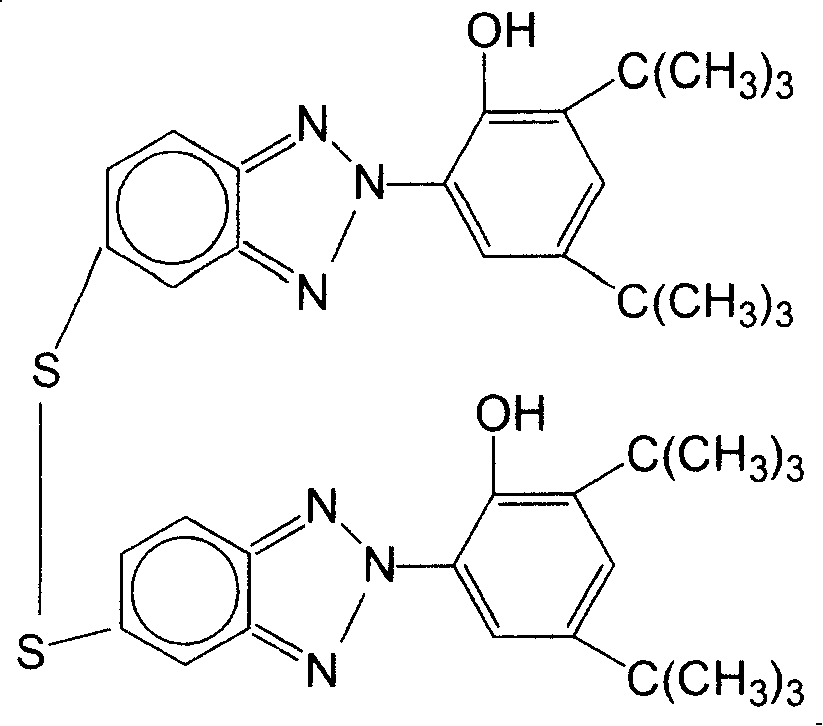
[0041] Add 30ml N-methylpyrrolidone (0.31mol) and 7.16 grams of 2-(2'-hydroxyl-3', 5'-di-tert-butylphenyl)-5-chlorobenzotriazole ( 0.01mol), heated to 186°C, and reacted for 18 hours. The solution was cooled to room temperature. Slowly add 7.5ml of water and 7.5ml of concentrated hydrochloric acid under stirring, a precipitate precipitates out after cooling, filter, wash the precipitate with petroleum ether, and dry to obtain 5,5'-dithio-bis[2-(2-hydroxy-3, 5-di-tert-butylphenyl)-2H-benzotriazole], yield 20%.
[0042] Physicochemical properties, spectral data and UV absorption of 5,5'-dithio-bis[2-(2-hydroxy-3,5-di-tert-butylphenyl)-2H-benzotriazole] prepared in this example (measure in chloroform) characteristic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com