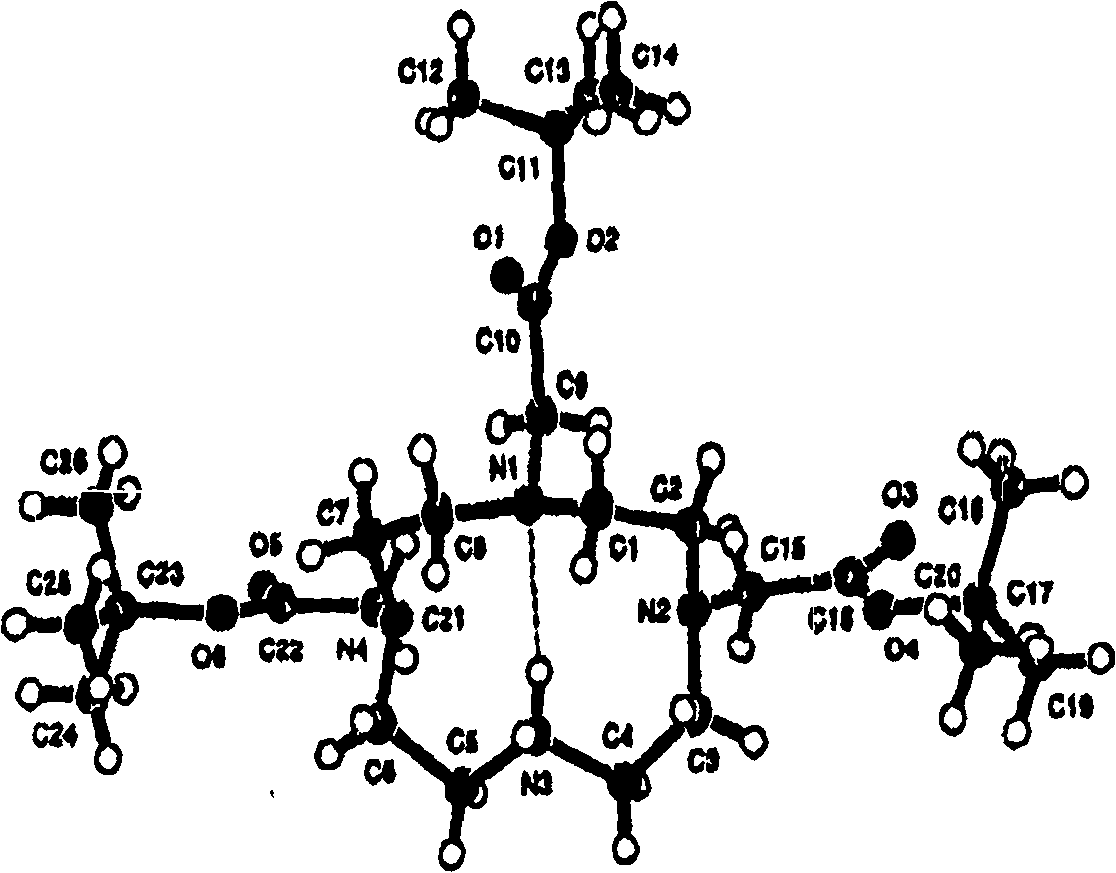
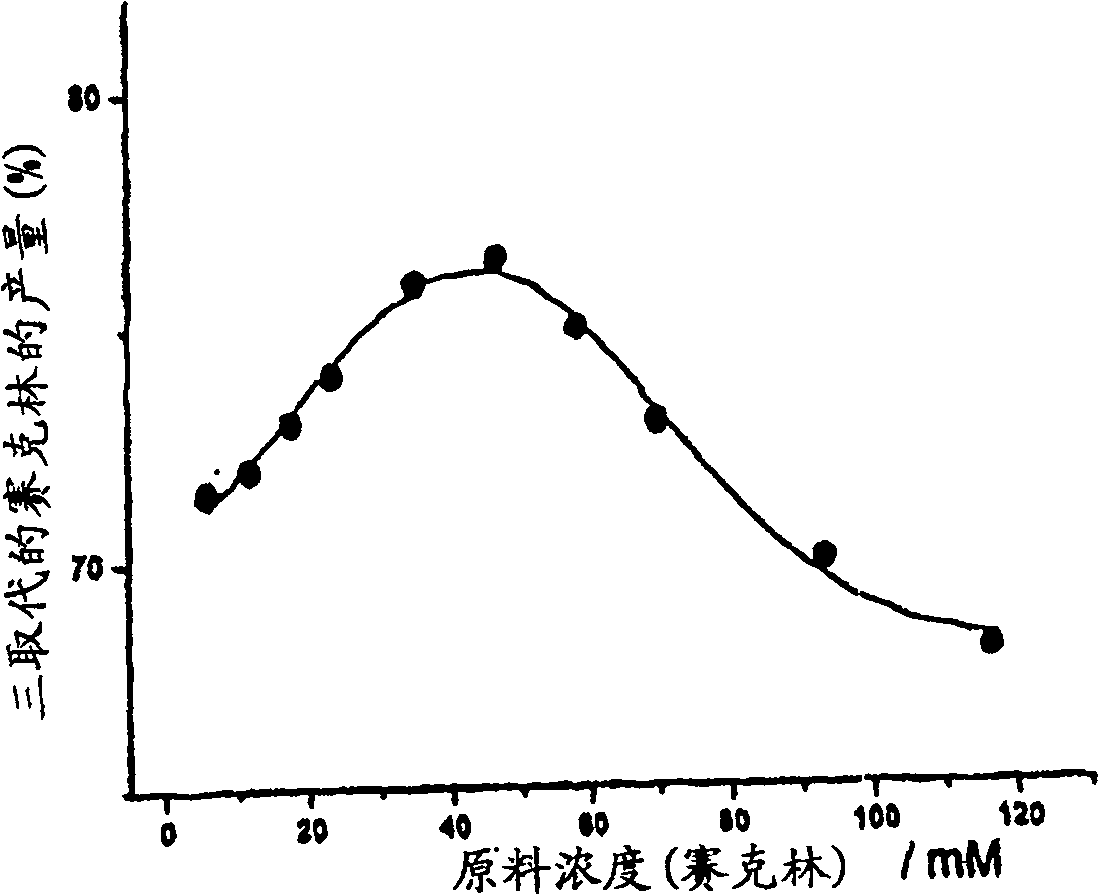
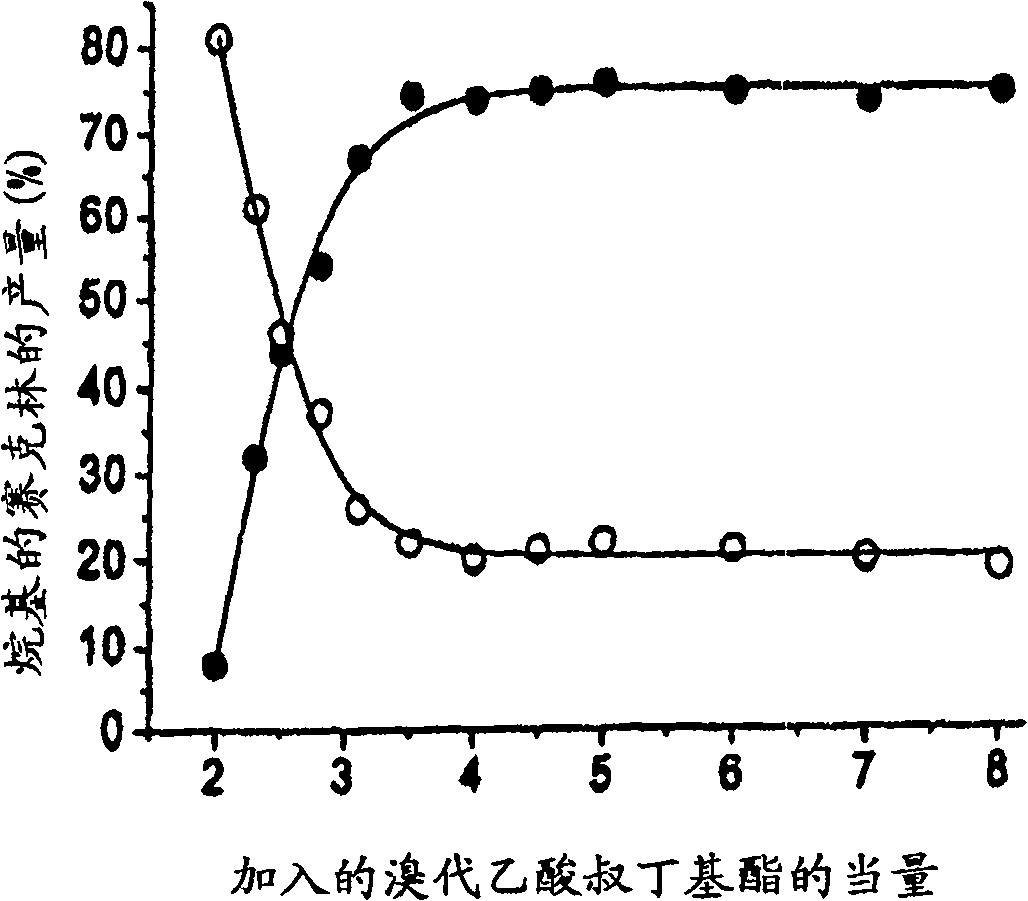
Synthesis of tris n-alkylated 1,4,7,10-tetraazacyclododecanes
A technology of tetraazacyclododecane, which is applied in the field of synthesis of tri-N-alkylated 1,4,7,10-tetraazacyclododecane, which can solve the problem of high labor costs and purification steps Trouble, time-consuming and other problems, to achieve the effect of effective response and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Tris-(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane (1)
[0033] Dissolve 3.3 equivalents of tert-butyl bromoacetate (773.0 mg, 7.6 mmol) in 10.0 mL of anhydrous chloroform, and add dropwise to 1,4,7,10-tetraazacyclododecane (Sikelin) ( 400.0 mg, 2.32 mmol) and 10.0 equivalents of triethylamine (2.3 g, 23.2 mmol) in a mixture of 40 mL of anhydrous chloroform, the reaction was carried out under an argon atmosphere for about half an hour, and the reaction mixture was stirred for 2 hours, and 0.5 equivalent anhydrous K 2 CO 3 , the solution obtained after 14 hours of reaction was washed with water (3×40 mL), washed with anhydrous Na 2 SO 4 The organic phase was dried and the solvent was removed under reduced pressure to give a clear oil. The crude product was purified by flash chromatography on alumina (dichloromethane / methane=200:5 (v / v), R f =0.35), to obtain tris-(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane (1) as a white powder...
Embodiment 2
[0034] Example 2 Tris-[(diphenyl)methylcarbamoylmethyl]-1,4,7,10-tetraazacyclododecane (2)
[0035] Dissolve 3.3 equivalents of N-2-chloroacetyl-diphenylmethylamine (1.98 g, 7.6 mmol) in 10.0 mL of anhydrous chloroform and add dropwise to 1,4,7,10-tetraazacyclododecane (Sikelin) (400.0mg, 2.32mmol) and 10.0 equivalents of triethylamine (2.3g, 23.2mmol) in a mixture of 40mL of anhydrous chloroform, the reaction was carried out for about half an hour under an argon atmosphere, and then the reaction was stirred The mixture was stirred for 2 hours, and 0.5 equivalent of anhydrous K was added 2 CO 3 , the solution obtained after 15 hours of reaction was washed with water (3×40 mL), washed with anhydrous Na 2 SO 4 The organic phase was dried and the solvent was removed under reduced pressure to give a bright yellow solid. Purify by flash chromatography on alumina (dichloromethane / methane=200:10 (V / V), R f = 0.30) crude product, tris-[(diphenyl)methylcarbamoylmethyl]-1,4,7,10-...
Embodiment 3
[0036] Example 3 Tris-[(R)-1-(1-phenyl)ethylcarbamoylmethyl]-1,4,7,10-tetraazacyclododecane (3)
[0037] Dissolve 3.3 equivalents of (R)-N-2-chloroacetyl-1-phenylethylamine (1.51 g, 7.6 mmol) in 10.0 mL of anhydrous chloroform and add dropwise to 1,4,7,10 tetraaza In a mixture of cyclododecane (Secline) (400.0 mg, 2.32 mmol) and 10.0 equivalents of triethylamine (2.3 g, 23.2 mmol) in 40 mL of anhydrous chloroform, the reaction was carried out under an argon atmosphere for about half an hour , and the reaction mixture was stirred for another 2 hours, and 0.5 equivalent of anhydrous K was added 2 CO 3 , the solution obtained after 14 hours of reaction was washed with water (3×40 mL), washed with anhydrous Na 2 SO 4 The organic phase was dried and the solvent was removed under reduced pressure to give a white solid. The crude product was purified by flash chromatography on alumina (dichloromethane / methane=200:12 (V / V), R f =0.25), to obtain three-[(R)-1-(1-phenyl)ethylcarb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com