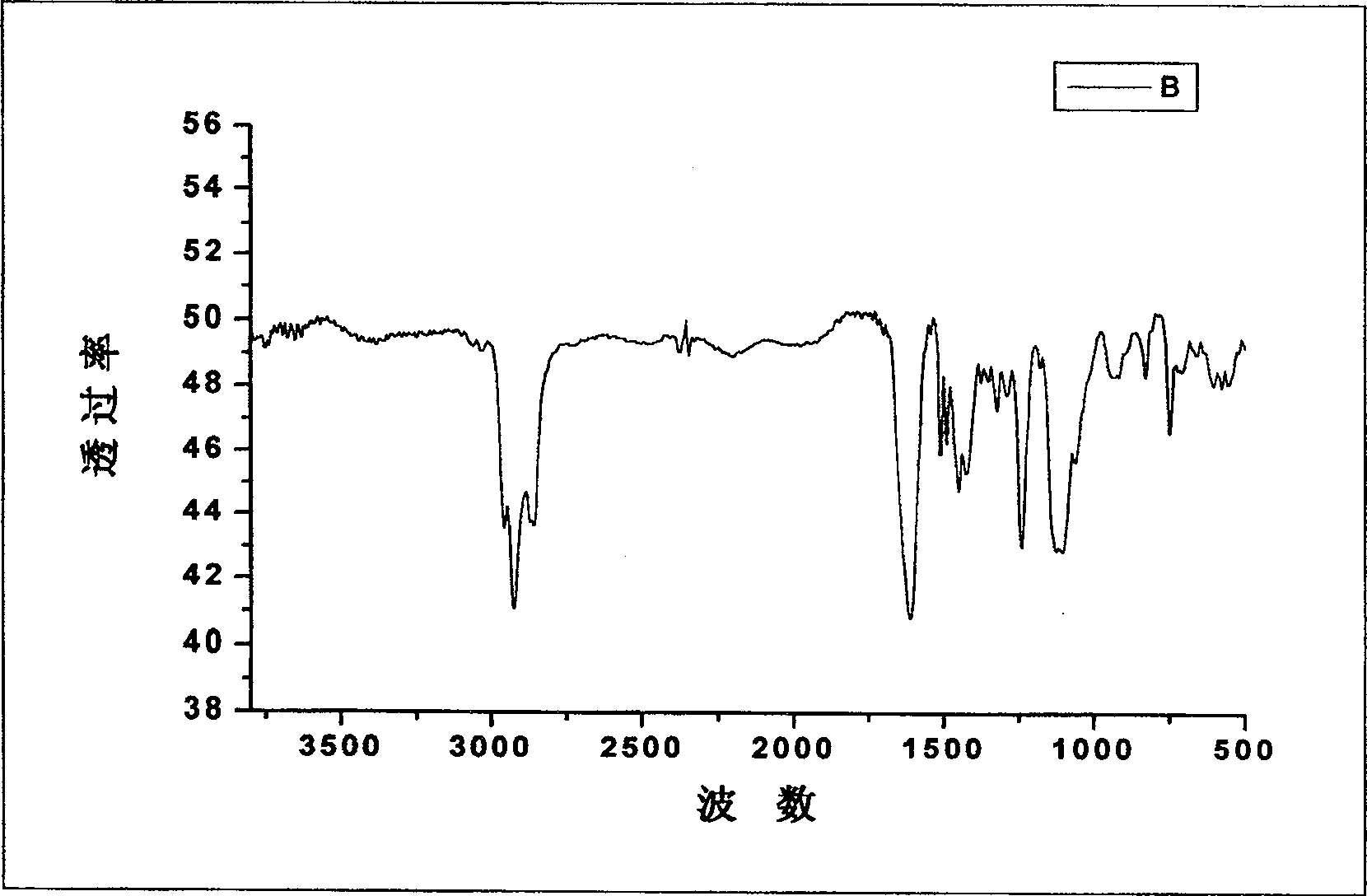
Alkyl- fragrant benzyl- polyethenoxy ether anionic surfactant and method of preparing the same and use thereof
A technology of alkyl arylbenzyl polyoxyethylene ether and alkyl arylbenzyl polyoxyethylene ether alcohol, which is applied in the field of alkyl arylbenzyl polyoxyethylene ether anionic surfactants and preparation thereof, and can solve the problem of unresolved problems. and alkyl arylbenzyl polyoxyethylene ether anionic surfactants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: the preparation of octyl benzyl trioxyethylene ether sodium acetate
[0066] (a) Preparation of Octylbenzyl Chloride
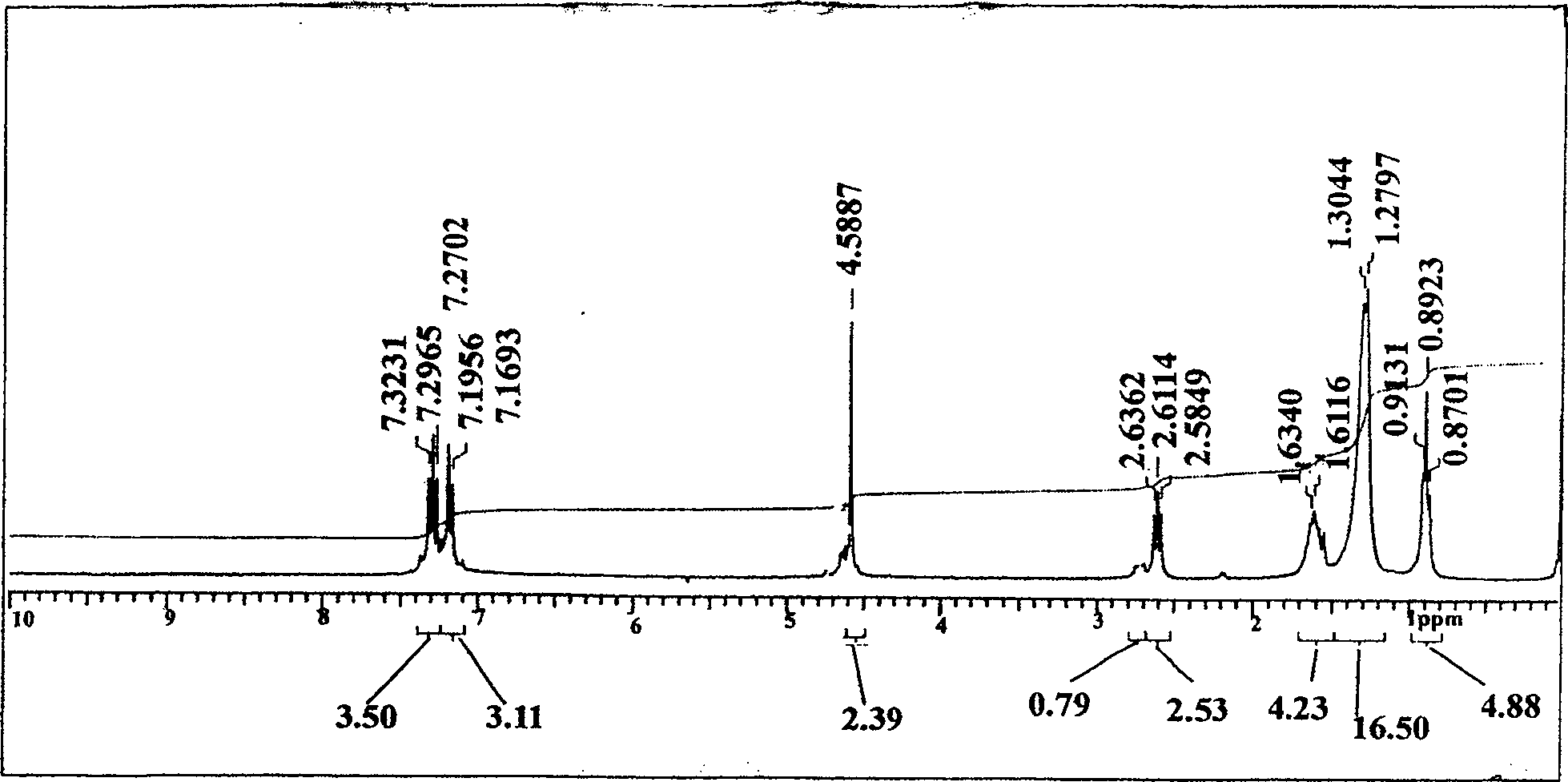
[0067] Put 0.5mol octylbenzene in the reaction kettle, then add 1mol paraformaldehyde and 0.35mol powdered anhydrous zinc chloride, add 110mL glacial acetic acid as solvent, stir vigorously and quickly pass in dry hydrogen chloride gas, at 70~ React at 80°C until hydrogen chloride is no longer absorbed, about 2 hours, cool to room temperature, separate the upper organic phase, and extract the lower acetic acid phase with petroleum ether for 2 to 3 times, combine the organic phases with 10wt% sodium carbonate and water in sequence Wash until neutral, dry with anhydrous sodium sulfate, add a little sodium bicarbonate to distill under reduced pressure, collect fractions at 165-167°C / 3.5mmHg, the yield is 75%, and it is a colorless liquid at room temperature. That 1 H NMR spectrum see figure 1 .
[0068] (b) Preparation of Octylbenzyl Trio...
Embodiment 2
[0076] Embodiment 2: Preparation of ammonium lauryl benzyl tetraoxyethylene ether sulfate
[0077] (a) Preparation of lauryl benzyl chloride
[0078] Put 0.5mol laurylbenzene in the reaction kettle, then add 0.9mol paraformaldehyde and 0.32mol powdered anhydrous zinc chloride, add 120mL glacial acetic acid as solvent, stir vigorously and quickly pass dry hydrogen chloride gas, at 85 React at ~90°C until hydrogen chloride is no longer absorbed, about 1.5 hours, cool to room temperature, separate the upper organic phase, and extract the lower acetic acid phase with petroleum ether for 2 to 3 times, combine the organic phases with 10wt% sodium carbonate and Wash with water until neutral, dry with anhydrous sodium sulfate, add a little sodium bicarbonate to distill under reduced pressure, collect fractions at 190-193°C / 3.5mmHg, the yield is 72%, and it is a white solid at room temperature.
[0079] (b) Preparation of lauryl benzyl tetraoxyethylene ether alcohol
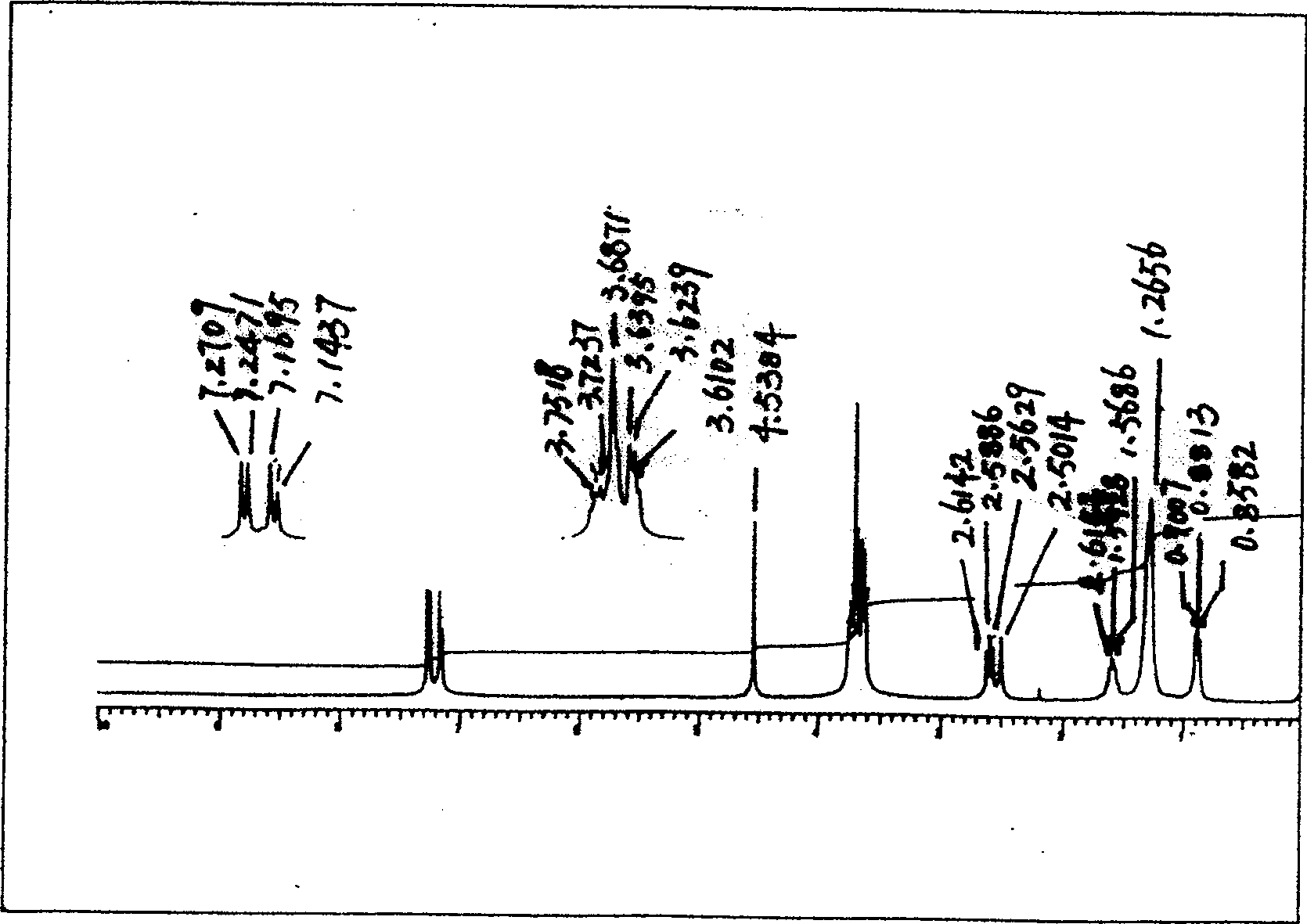
[0080] Get the ...
Embodiment 3
[0084] Embodiment 3: the preparation of sodium octyl benzyl trioxyethylene ether propyl sulfonate
[0085] (a) Preparation of Octylbenzyl Chloride
[0086] Put 0.5mol octylbenzene in the reaction kettle, then add 1mol paraformaldehyde and 0.35mol powdered anhydrous zinc chloride, add 110mL glacial acetic acid as solvent, stir vigorously and quickly pass in dry hydrogen chloride gas, at 70~ React at 80°C until hydrogen chloride is no longer absorbed, about 2 hours, cool to room temperature, separate the upper organic phase, and extract the lower acetic acid phase with petroleum ether for 2 to 3 times, combine the organic phases with 10wt% sodium carbonate and water in sequence Wash until neutral, dry with anhydrous sodium sulfate, add a little sodium bicarbonate to distill under reduced pressure, collect fractions at 165-167°C / 3.5mmHg, the yield is 75%, and it is a colorless liquid at room temperature.
[0087] (b) Preparation of Octylbenzyl Trioxyethylene Ether Alcohol
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com