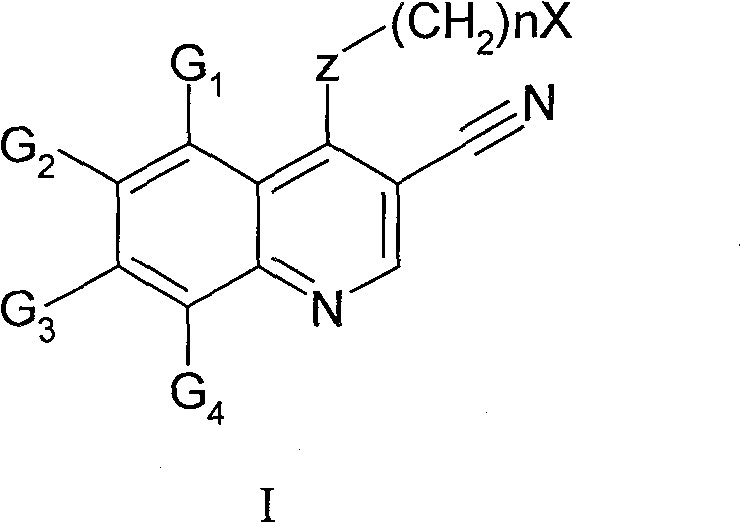
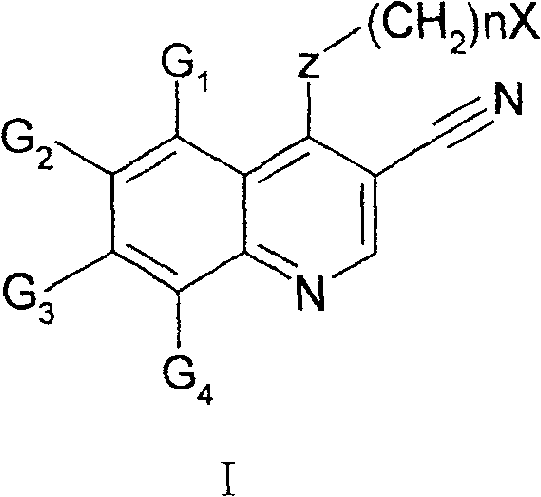
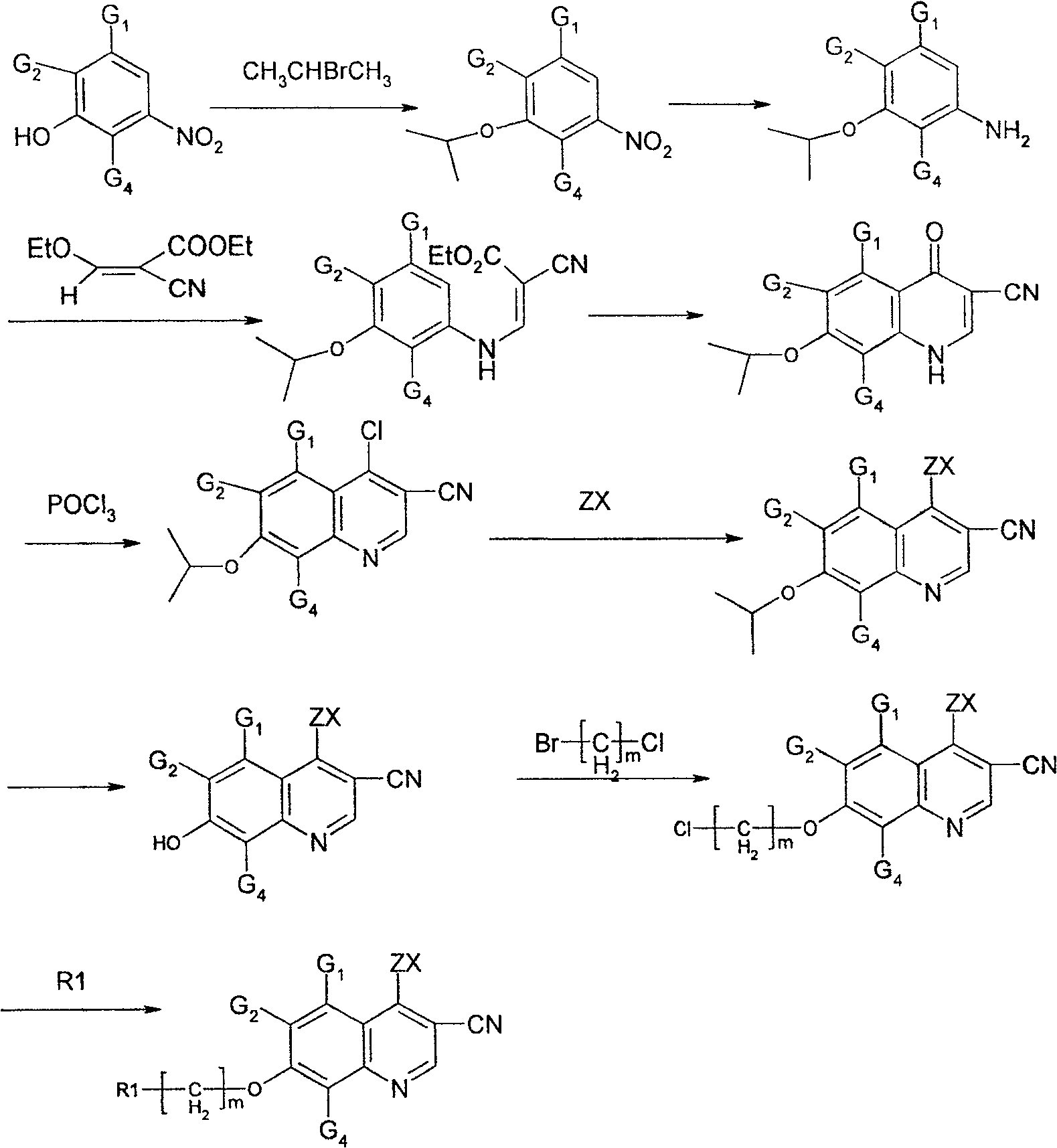
Quinoline derivative, preparation method and medical use thereof
A derivative, quinoline technology, applied in the field of treating tumor diseases, can solve problems such as signal transduction disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] 3-Isopropoxy-4-methylnitrobenzene (1)
[0114] Add 60g (0.39mol) of 3-hydroxy-4-methylnitrobenzene and 100g (0.72mol) of potassium carbonate into a 500ml three-necked flask, dissolve in 300ml of acetone, stir mechanically, and slowly add 50ml of 2-bromopropane ( 0.50mol), react at 40°C for 6 hours. Then the reaction liquid was poured into 1000 ml of water, extracted three times with chloroform, the extract was concentrated, and vacuum-dried to obtain 72.2 g of a brownish-yellow liquid product with a yield of 95%. Molecular formula: C10H13NO3, molecular weight: 195.22
[0115] MS (EI) m / z 195.
Embodiment 2
[0117] 3-isopropoxy-4-methylaniline (2)
[0118] Add 41g (0.73mol) of iron powder, 200ml of water, 50ml of acetic acid and 50ml of ethanol into a 500ml three-neck flask, stir and heat to 30°C for 20min, then slowly add 3-isopropoxy 4-methylnitro Benzene 60g (0.31mol), reacted at 50°C for 2 hours, filtered with suction, combined the filtrates, extracted 3 times with chloroform, concentrated the extract, and dried in vacuo to obtain 45g of a brown oily liquid. Yield 89%. Molecular formula: C10H15NO, molecular weight: 165.24.
[0119] MS (EI) m / z 165.
Embodiment 3
[0121] (Z)-2-Nitryl-3-(3-isopropoxy-4-methylphenyl-amino)-acrylic acid ethyl ester (3)
[0122] Add 45g (0.27mol) of 3-isopropoxy-4-methylaniline, 51g (0.30mol) of (Z)-3-ethoxy-2-nitrile-acrylic acid ethyl ester and 160ml of toluene into a 250ml three-necked flask , heated and stirred, refluxed for 4 hours, cooled to room temperature, a large amount of white solid was precipitated, filtered with suction, recovered toluene in the filtrate, and the filter cake was recrystallized with ethanol to obtain 72 g of the product with a yield of 93%. Molecular formula: C16H20N2O3, molecular weight: 288.35. Mp: 138°C.
[0123] MS (EI) m / z 288.
[0124] 1 H-NMR (AV-500, δ, DMSO-d 6 ): 1.21-1.28(m, 9H); 2.08(s, 3H); 4.14-4.26(t, 2H); 4.55-4.57(m, 1H); 6.81-6.89(m, 2H); 7.01-7.02(d , 1H); 8.26-8.39(t, 1H); 10.58-10.68(d, 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com