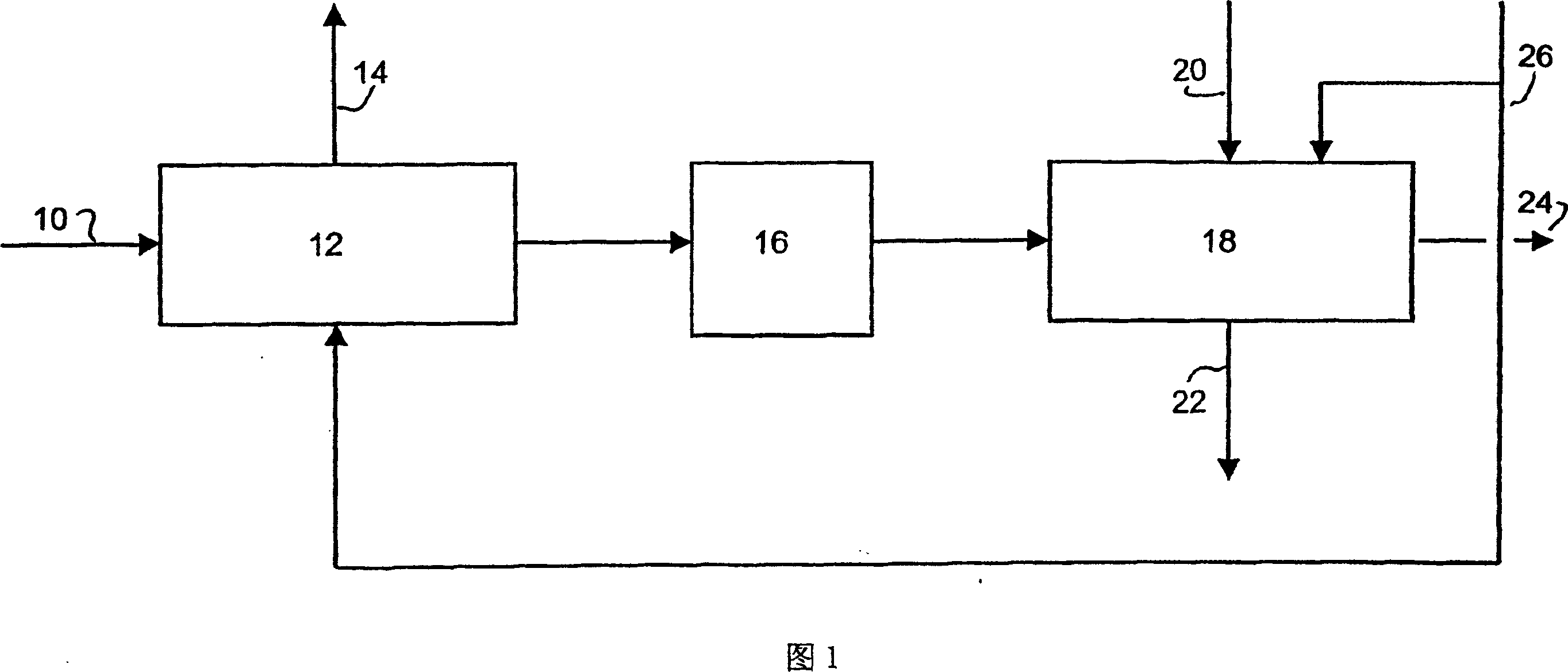
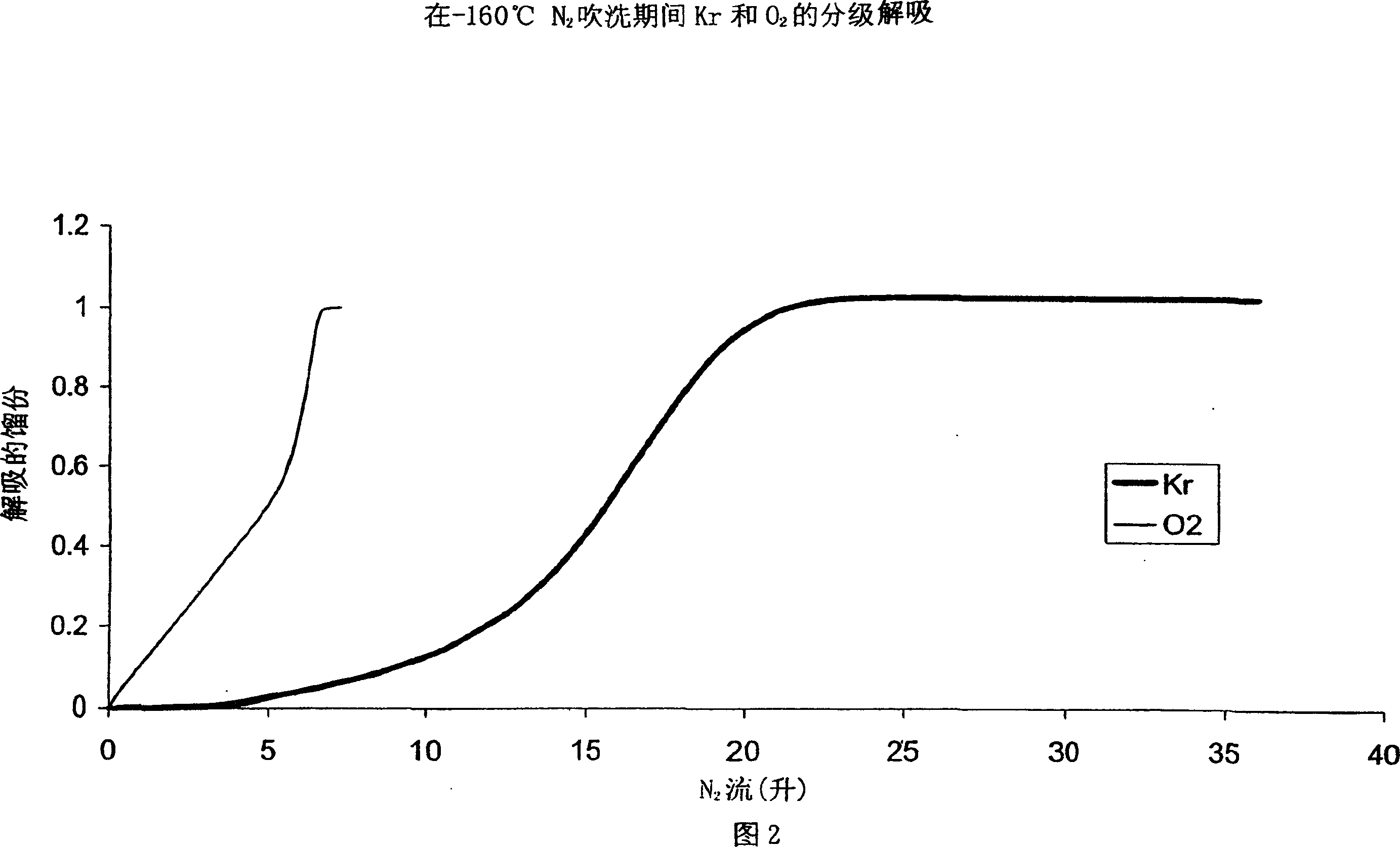
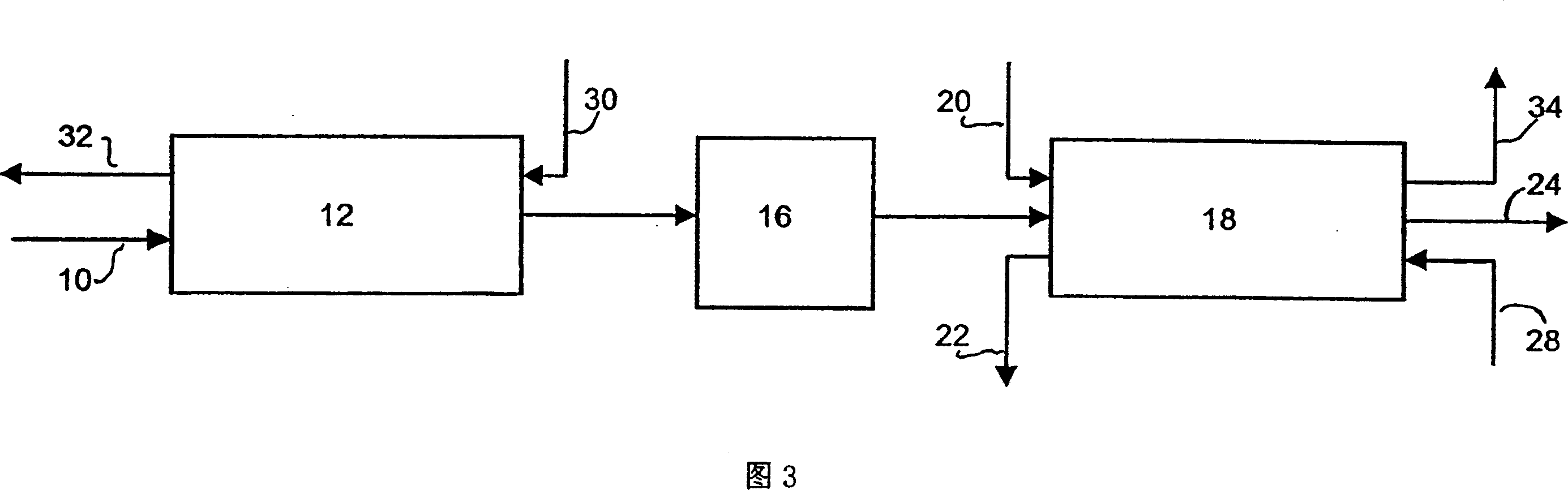
Process and adsorbent for the recovery of krypton and xenon from a gas or liquid stream
A technology of adsorbent and adsorbent bed, applied in chemical instruments and methods, separation methods, liquefaction, etc., can solve the problem of low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Oxygen and xenon adsorption isotherms were measured at 30°C on silica gel, 13X and 20% AgLiLSX. The results are listed in Table 1, which gives the Henry's law constants (starting isotherm slopes) for krypton, xenon and oxygen. The table also gives the selectivity to xenon relative to oxygen expressed as a ratio of its Henry's law constants. The silica is Grace Davison grade 40 (750m 2 / g), 13X is an APG grade from UOP and 20% AgLiLSX is produced by ion exchange of silver(I) into commercially available LiLSX.
[0083] Table 1
[0084] adsorbent
[0085] The results in Table 1 show that the introduction of silver(I) ions into the zeolite structure will greatly increase the capacity of krypton (19 times greater than 13X) and xenon (62 times greater than 13X). The table also shows that the Ag-exchanged zeolite has a particularly high xenon / oxygen selectivity. Finally, the selectivity and capacity of the silver-exchanged zeolite will be much higher than that of...
Embodiment 2
[0087] The low temperature adsorption rate of xenon from liquid argon (LAR) was measured on silica gel (Grace Davison B-411) and 40% AgLiLSX. Liquid argon containing 20 ppm of xenon was fed into an adsorber 2 inches in diameter and 20 inches in length until the xenon was completely penetrated. Liquid argon (LOX) is used as the cryogen for safety reasons in the use of liquid oxygen (LOX), and it is expected that the performance of LAR and LOX should be substantially the same. The flow rate into the adsorber is approximately 2001bmoles / ft 2 / hr(53.4kgmoles / m 2 / sec). The results of the penetration test are listed in Table 2.
[0088] Table 2
[0089] adsorbent
[0090] The results in Table 2 show that 20% AgLiLSX is a much improved adsorbent for selective adsorption of xenon from cryogenic liquids than silica gel (prior art adsorbent).
Embodiment 3
[0092] The xenon capacity of 20% AgLiLSX was determined by measuring breakthrough curves at -78°C (195K) and -160°C (113K) with 20 ppm xenon in argon at a feed pressure of 50 psig (345 kPa). The column used was 5 cm long and 3 / 8 inch (32 mm) in diameter. The test results compared with the results obtained in Example 2 are listed in Table 3.
[0093] table 3
[0094] adsorbent
[0095] The results in Table 3 are unexpected since the adsorption capacity of Xe passes a maximum value with temperature. The results also show that the recovery of the noble gas in the vapor phase is preferably performed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com