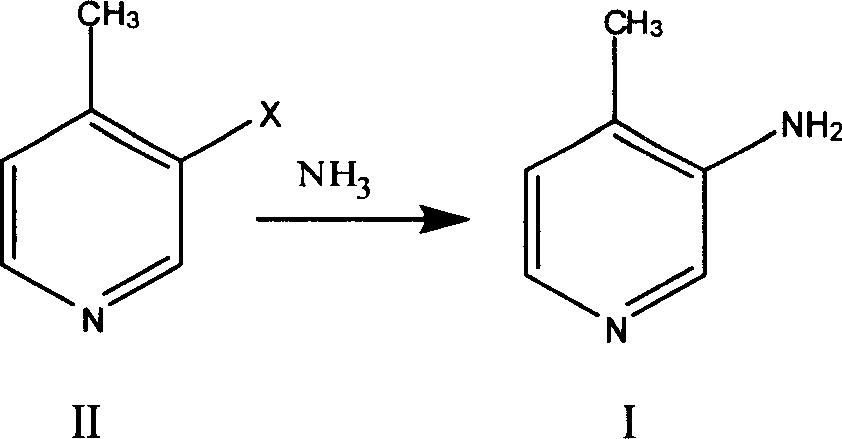
Preparation process of 3 amino-4 methyl pyridine
A technology of picoline and amino, which is applied in the field of preparation of intermediate 3-amino-4-picoline, can solve the problems of inability to realize industrial production and harsh reaction conditions, and achieve the effect of reducing production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of compound II:
[0020] Add 720ml of 20% oleum and 140g of 4-picoline into the reaction kettle, raise the temperature to 160°C, slowly add 250g of bromine dropwise, react at 160-170°C for 15 hours, cool the reaction liquid to room temperature, pour it into ice water, Adjust the pH to 10-11 with sodium carbonate, and steam distill to obtain 171 g of 3-bromo-4-methylpyridine with a yield of 66%.
Embodiment 2
[0022] Preparation of compound I:
[0023] Add 300ml of methanol, 150g of 3-bromo-4-picoline, 5g of copper sulfate into the autoclave, feed ammonia gas until the pressure reaches 5atm, heat to 160°C, react for 8 hours, cool, filter with suction, and concentrate the filtrate under reduced pressure , the obtained solid was recrystallized from ethyl acetate to obtain 89 g of 3-amino-4-picoline (I), with a yield of 95%.
Embodiment 3
[0025] Preparation of compound I:
[0026] Add 500ml of concentrated ammonia water, 150g of 3-bromo-4-picoline, and 5g of copper sulfate into the autoclave, heat to 180°C after airtight, react for 8 hours, cool, extract 3 times with 500ml of dichloromethane, concentrate under reduced pressure, 84 g of 3-amino-4-picoline (I) was obtained, and the yield was 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com