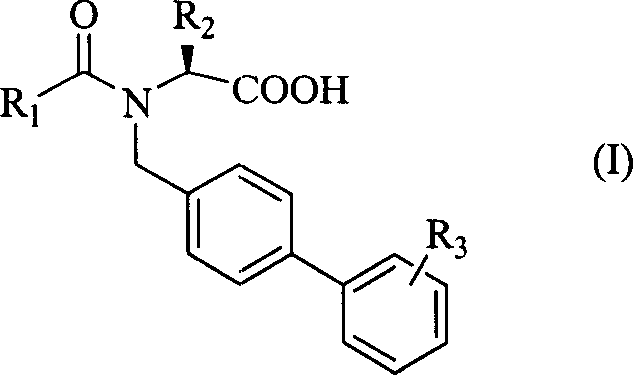
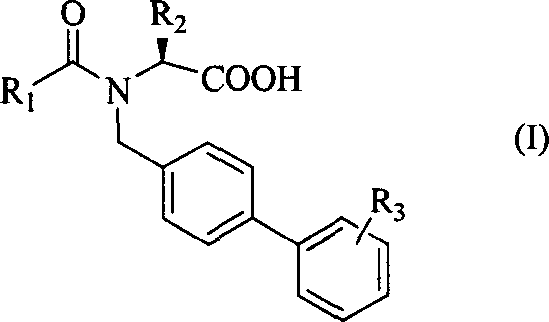
Amino acid diphenyl compound
A compound and amino acid technology, applied in the field of medicinal chemistry, can solve problems such as explosives, shortened existence time of compounds, toxicity, etc., and achieve good preventive or therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] [Example 1] (S)-N-n-butyryl-N-[4-(2-(5-oxo-1,2,4-oxadiazole-3)phenyl)]benzylisobright amino acid
[0029] Step 1: (L)-Isoleucine Methyl Ester Hydrochloride
[0030] Add 5 mL of anhydrous methanol to a 50 mL three-necked flask under ice-bath conditions, stir, slowly add 1 mL of thionyl chloride dropwise, after the dropwise addition, stir for 30 min, add 1 g (L)-isoleucine, and stir overnight, The solvent was evaporated under reduced pressure. Recrystallized from methanol-ether to obtain 1.4 g of a colorless needle-like solid, mp: 153-160°C.
[0031] Step 2: N-[4-(2-cyanophenyl)]benzyl-L-isoleucine methyl ester
[0032] in N 2 Under protective conditions, dissolve 2.0g (L)-isoleucine methyl ester hydrochloride in 15mL DMF, cool in an ice bath, stir, add 5mL triethylamine dropwise, and then add 3.0g 2'-cyano-4 - Bromomethylbiphenyl. React at 70°C and monitor by TLC. After the reaction is complete, cool down rapidly, add 15 mL of distilled water, and extract with ethy...
Embodiment 2
[0043] [Example 2] (S)-N-n-butyryl-N-[4-(2-(5-oxo-1,2,4-oxadiazole-3)phenyl)]benzylbenzenepropane amino acid
[0044] The experimental procedure is as described in Example 1, and the yield is 89.4%. 1 H-NMR (CDCl 3, 500.12MHz, doubling due to amide rotamers) δ: 7.61-7.41 (13H, m, Ph-H), 4.43 (d, 1H, J=14.2Hz, -N-CH-), 4.30-4.27 (m), 3.63(d, J=16.3Hz, -CH 2 -N-), 3.27(d, 2H, J=7.8Hz, -CH-C H 2 -Ph), 2.31-2.27(m, 2H, -CO-CH 2 -), 1.31-1.28 (m, 2H, -CH 2 -C H 2 -CH 3 ), 0.91-0.85 (m, 3H, -CH3); MS (m / z): 486.1 [M+1] + , 508.2[M+Na] + .
Embodiment 3
[0045] [Example 3] (S)-N-n-pentanoyl-N-[4-(2-(5-oxo-1,2,4-oxadiazole-3)phenyl)]benzylbenzenepropane amino acid
[0046] The experimental procedure is as described in Example 1, and the yield is 89.1%. 1 H-NMR (CDCl 3 , 500.12MHz, doubling due to amide rotamers) δ: 7.77-7.16 (13H, m, Ph-H), 4.42 (d, J=16.3Hz), 4.28 (m, -N-CH 2 -), 3.64(d, 1H, J=17.1Hz, -CH-N-), 3.31-3.24(m, 2H, -CH-C H 2 -Ph), 2.34-2.24(m, 2H, -CO-CH 2 -), 1.58-1.53 (m, 2H, -CH 2 -C H 2 -CH 2 ), 1.39-132 (m, 2H, -CH 2 -C H 2 -CH 3 ), 0.91-0.84 (m, 3H, -CH 3 ); MS(m / z): 500.1[M+1] + , 522.1[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com