Process for preparing micronized medicine by using micro-reactor
A technology of micro-reactors and medicines, which is applied in the direction of making medicines into special physical or ingestible devices, powder delivery, etc., which can solve the problems of inconvenient emulsifier processing, high energy consumption, and increased raw material costs, and achieve continuous The effects of reaction stability, particle uniformity, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
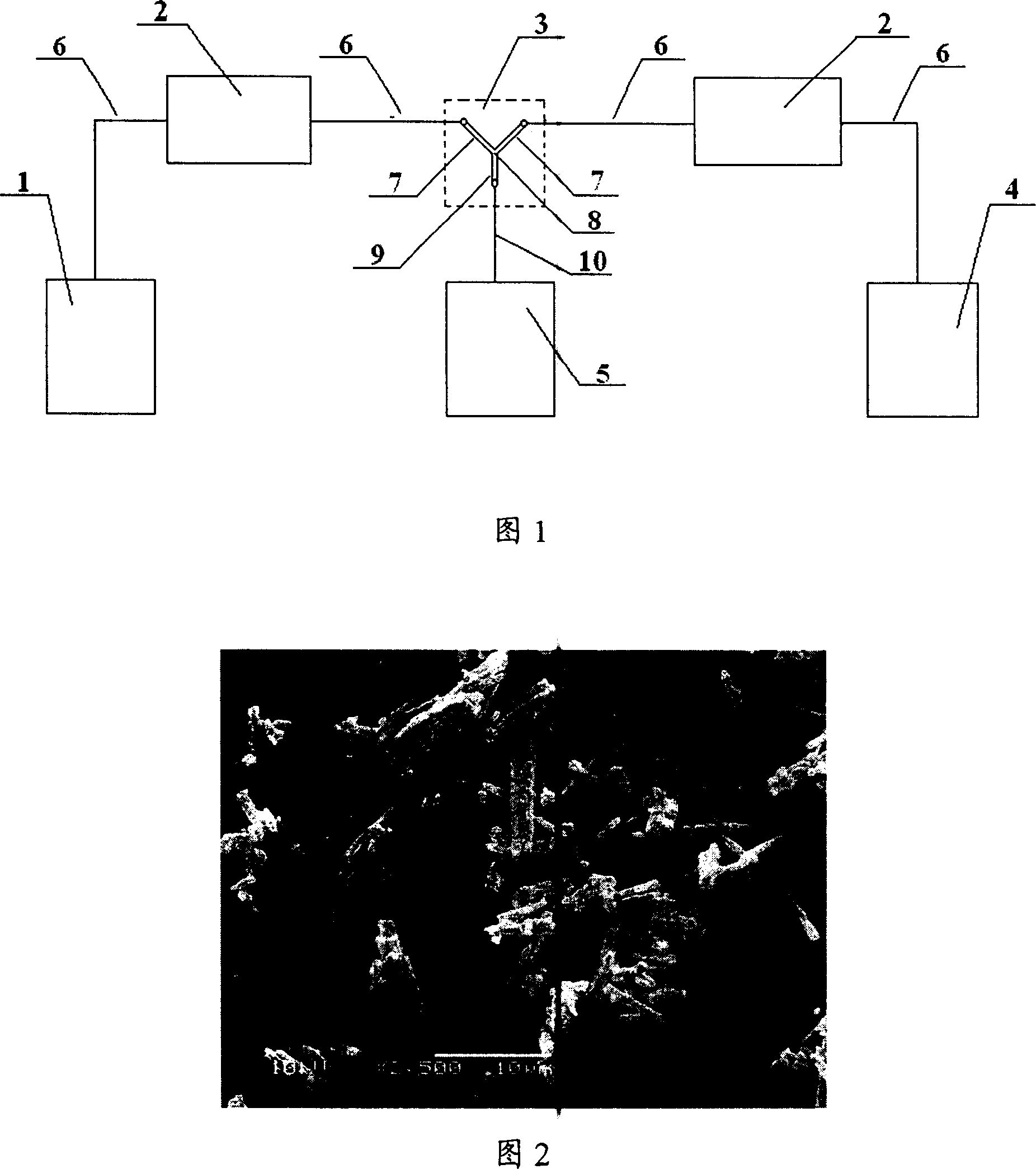
[0034] The reaction device that adopts is as shown in Figure 1, and bulk drug solution storage bottle 1 is connected with the advection pump 2 that is used to transport bulk drug solution by capillary line 6, and anti-solvent storage bottle 4 is passed through the advection pump 2 that is used to transport anti-solvent Capillary line 6 is connected, and microreactor 3 is connected with two advection pumps 2 respectively by capillary line 6, and in microreactor 3, the pipeline 7 that is used to transport bulk drug solution passes through capillary line 6 and is used for transporting bulk drug solution. The advection pump 2 is connected, the pipeline 7 for transporting the anti-solvent is connected with the advection pump 2 for transporting the anti-solvent through the capillary line 6, the angle between the pipeline 7 for transporting the bulk drug solution and the pipeline 7 for transporting the anti-solvent 60°, a microspace 8 is formed between the outlets of the two pipelines...
Embodiment 2
[0037] The microreactor that adopts is identical with embodiment 1.
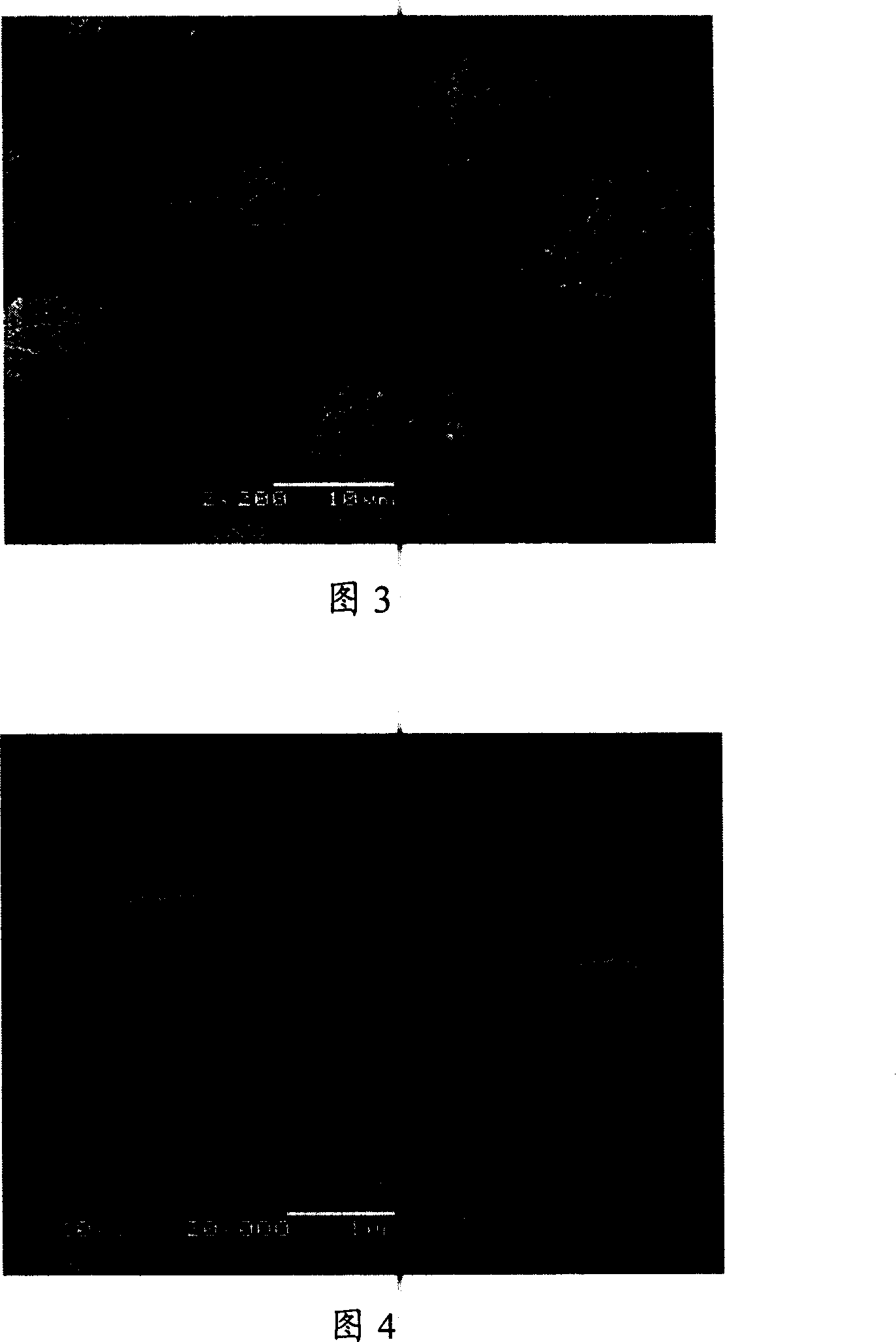
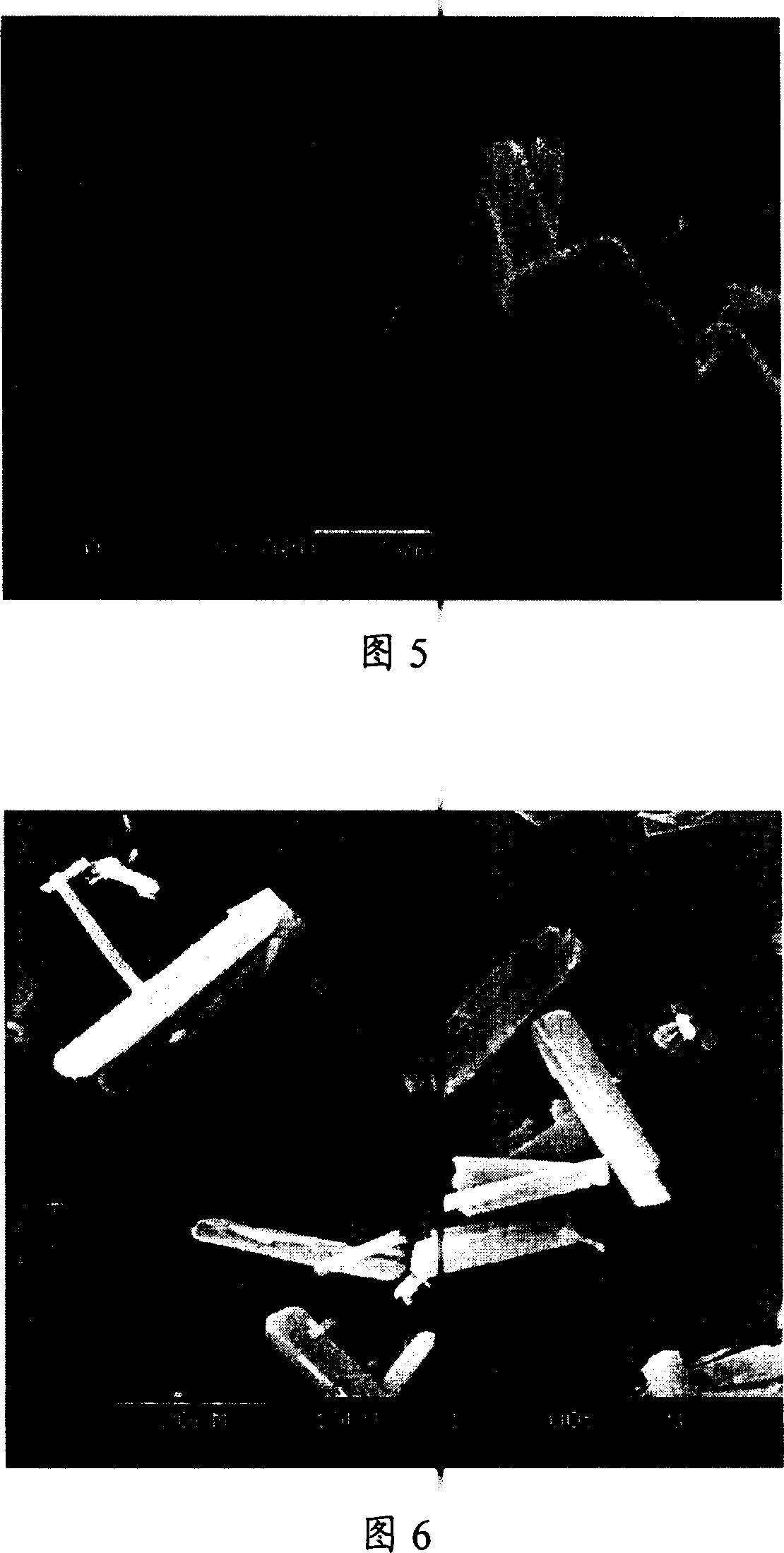
[0038] Take by weighing azithromycin bulk drug 4.0g, be dissolved in the ethanol of 20 ℃ and be mixed with the azithromycin ethanol solution 50ml that concentration is 0.08g / ml (being about 20% of the saturated concentration of azithromycin in ethanol under 20 ℃), place in raw material In the medicine solution storage bottle, take 500ml deionized water in the anti-solvent storage bottle as the anti-solvent. Azithromycin ethanol solution and deionized water are introduced into the microreactor through the solution inlet and the antisolvent inlet by using a parallel flow pump, and the two fluids intersect at the intersection of the microchannel and are mixed rapidly and completely, and the resulting product slurry is discharged from the Mouth collection. The feed rates of azithromycin solution and water were 8ml / min and 80ml / min, respectively, and the recrystallization temperature was 20°C. The recrystallize...
Embodiment 3
[0040] The microreactor that adopts is identical with embodiment 1.
[0041] Take by weighing 1.0g of danazol crude drug, dissolve it in 20 DEG C of ethanol and prepare the danazol ethanol solution with a concentration of 0.02g / ml (about 92% of the saturation concentration of danazol in ethanol at 20 DEG C) 50ml, placed in the crude drug solution storage bottle, and 500ml deionized water was placed in the anti-solvent storage bottle as the anti-solvent. The danazol ethanol solution and the deionized water are introduced into the microreactor substantially simultaneously through the solution inlet and the anti-solvent inlet respectively by using an advection pump, and the two fluids intersect at the intersection of the microchannel and are mixed rapidly and completely, and the resulting product slurry is obtained from The outlet is collected. The feed rates of the danazol solution and water were 7ml / min and 70ml / min, respectively, and the recrystallization temperature was 20°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com