Dispersible tablet of pidotimod and its preparing process and use
A technology of pidotimod and dispersible tablets, which is applied in the pharmaceutical field to achieve the effects of good dispersion state, fast absorption and rapid drug dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
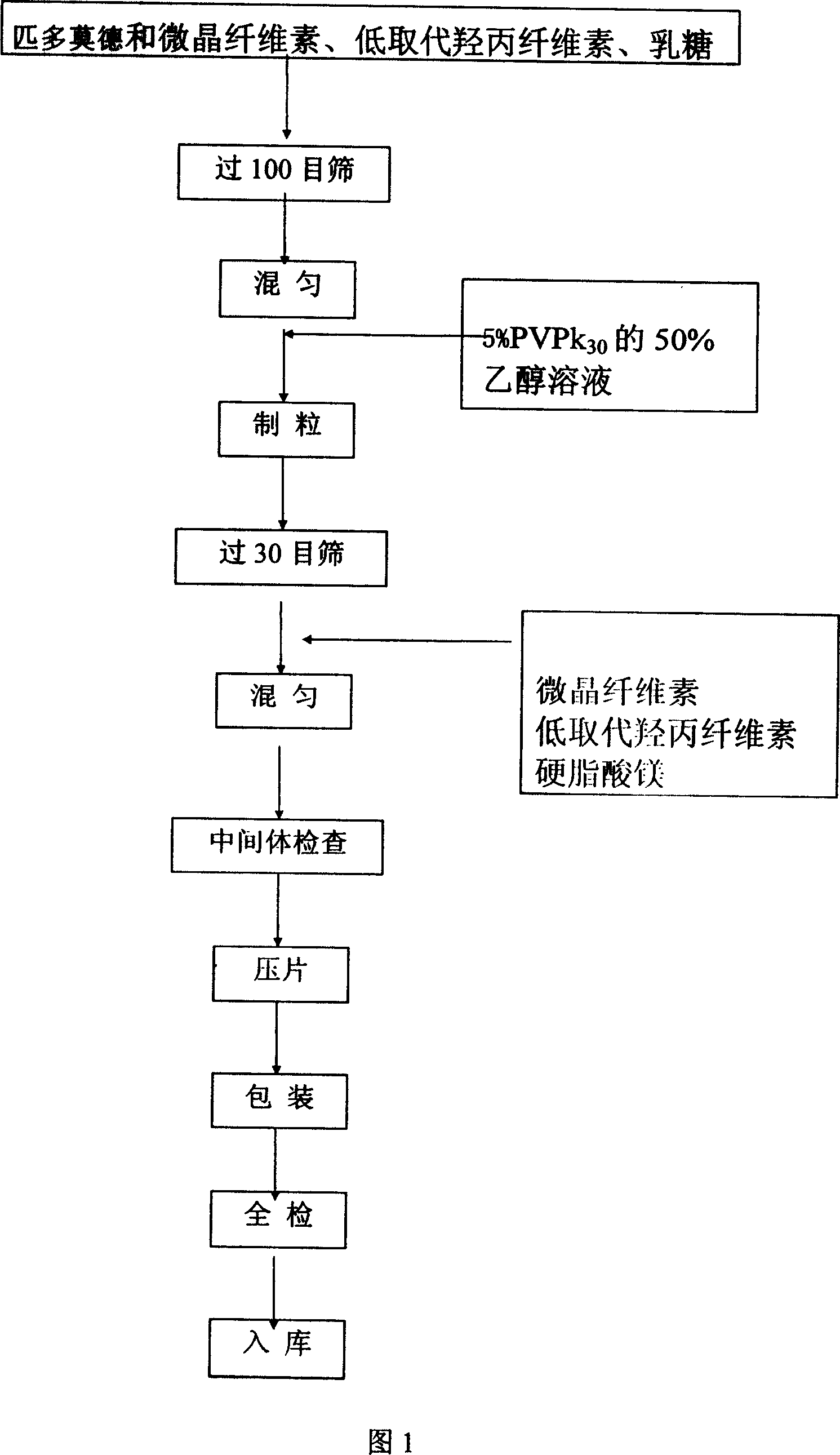
[0051] Embodiment 1: the preparation method of pidotimod dispersible tablet
[0052] (1) According to the optimal prescription quantity provided by the present invention, all raw materials and auxiliary materials are accurately weighed, and the raw materials are pulverized through a 100 mesh sieve; when making wet granules, pass through a 30 mesh sieve and granulate with a 30 mesh sieve.
[0053] (2) Microcrystalline cellulose and low-substituted hydroxypropyl cellulose disintegrants and fillers are used, and the method of internal and external addition is used to improve the disintegration speed of the tablet and the dissolution rate of pidotimod. Crystalline cellulose and low-substituted hydroxypropyl cellulose are divided into internally added and externally added parts, and the ratio of internally added to externally added is 2:1 by weight.
[0054] (3) Use a 50% ethanol solution of polyvinylpyrrolidone as an adhesive, and the concentration is generally 2% to 10%.
[0055...
Embodiment 2
[0060] Embodiment 2: the preparation of pidotimod dispersible tablet
[0061]1) Accurately weigh all raw materials and auxiliary materials according to the optimal prescription quantity provided by the present invention, the raw materials pass through a 120-mesh sieve, and the auxiliary materials pass through a 100-mesh sieve; wherein microcrystalline cellulose and low-substituted hydroxypropyl cellulose are divided into internally added and The external part, microcrystalline cellulose and low-substituted hydroxypropyl cellulose internal and external parts are weighed separately, and marked; the ratio of microcrystalline cellulose and low-substituted hydroxypropyl cellulose internal and external parts is 3 : 1, weight ratio;
[0062] 2) Put the pidotimod raw materials and all the internally added auxiliary materials in a mixer and mix them uniformly; add an appropriate amount of 50% ethanol solution of 5% polyvinylpyrrolidone, continue mixing, and make a suitable soft materia...
Embodiment 3
[0066] Embodiment 3: Orthogonal experiment
[0067] Get 40g of pidotimod and pharmaceutical excipients (excipients refer to microcrystalline cellulose, low-substituted hydroxypropyl cellulose, polyvinylpyrrolidone), with the amount of microcrystalline cellulose, low-substituted hydroxypropyl cellulose, polyethylene The concentration of pyrrolidone and the ratio of internal and external excipients (the ratio of internal and external excipients is 2:1) were factors, and three levels were designed for screening. The results are shown in Table 1.
[0068] Table 1. Four-factor three-level table for prescription optimization
[0069]
[0070] Wherein, the amount of microcrystalline cellulose and low-substituted hydroxypropyl cellulose is the total weight, and the ratio of internal and external auxiliary materials is 2:1.
[0071] Get pidotimod and auxiliary materials, cross 100 mesh sieves, according to the orthogonal test table, accurately weigh all raw materials, wherei...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com