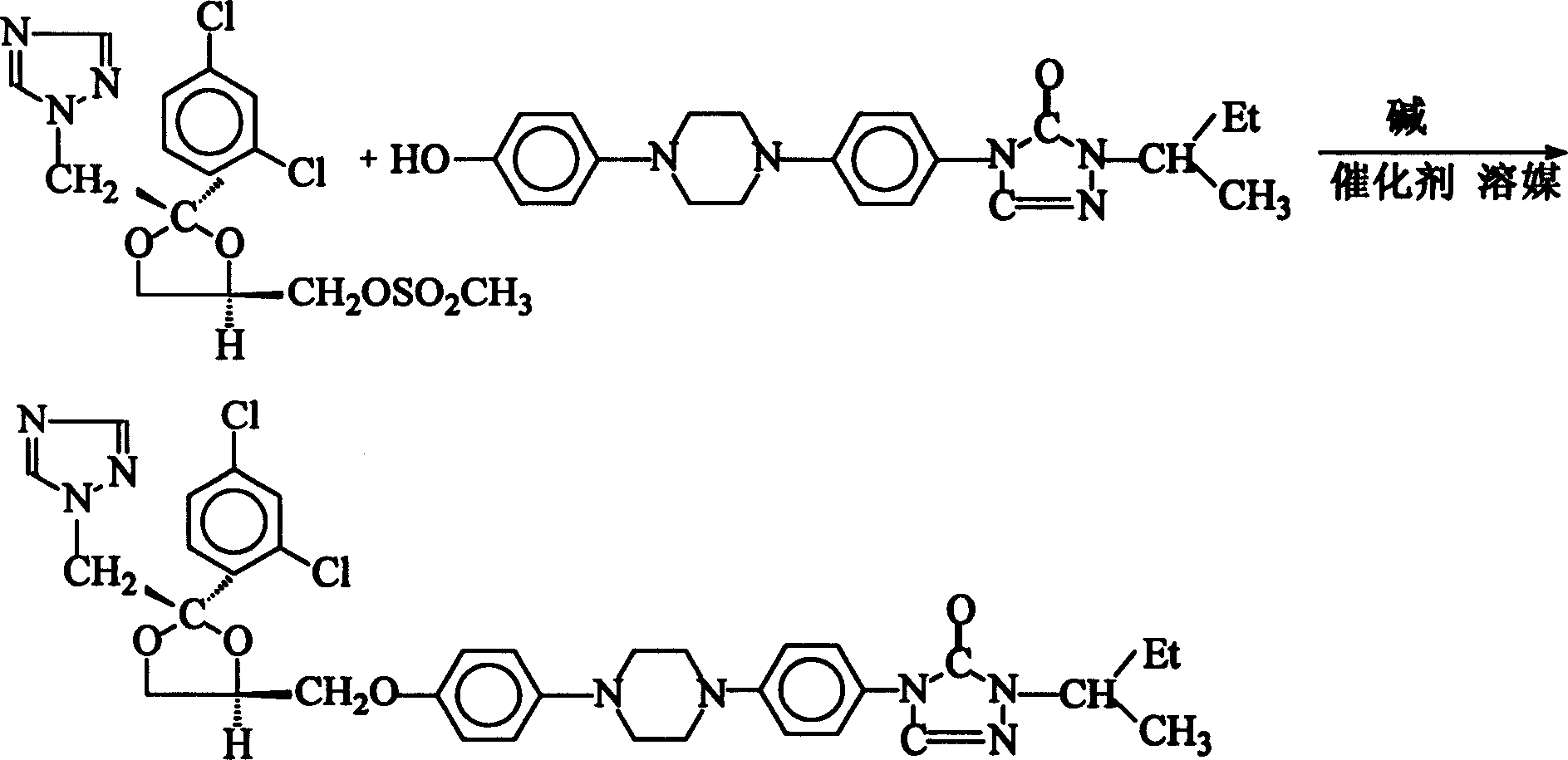
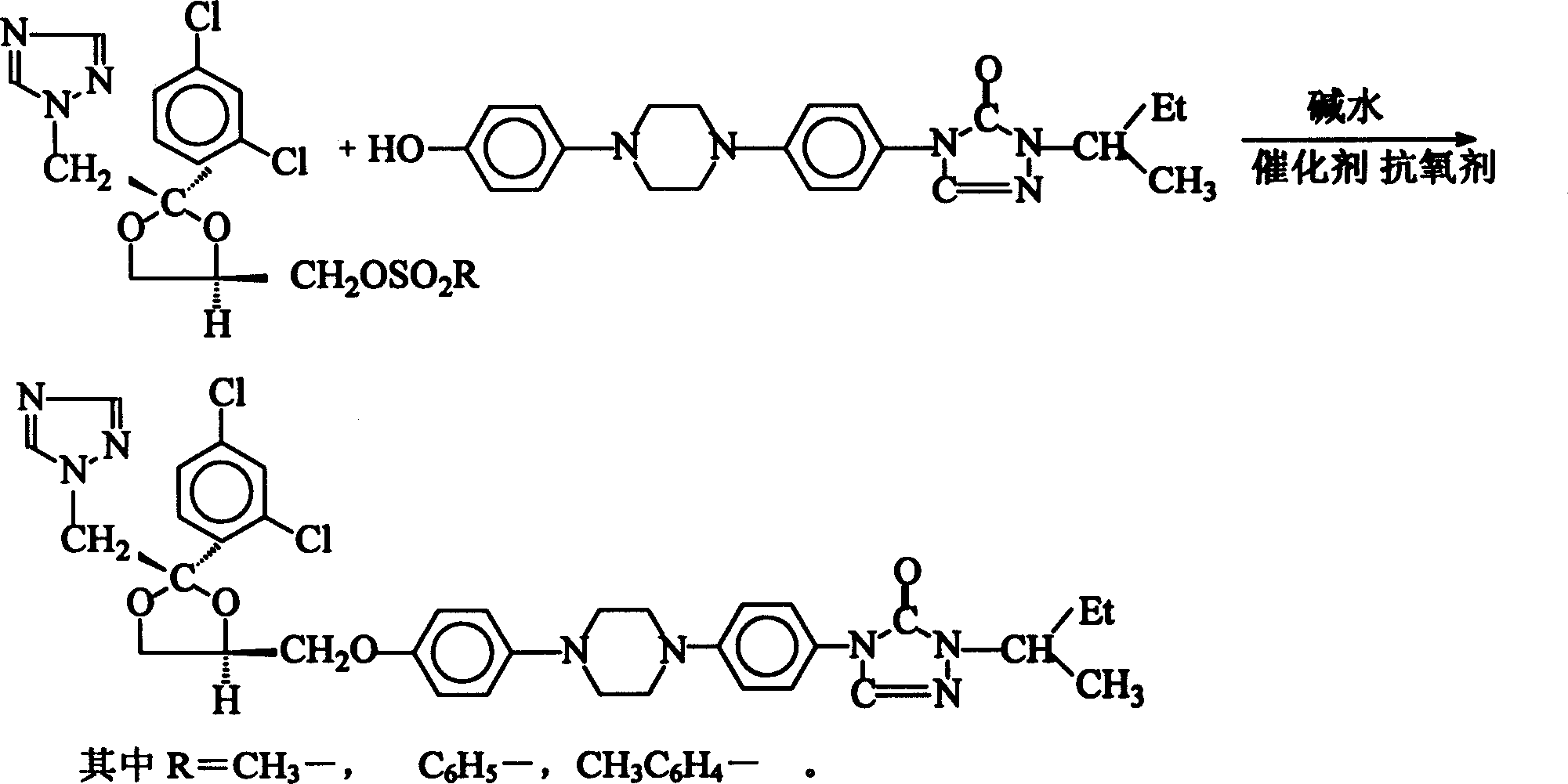
Method of synthesizing Itraconazole
A synthetic method and the technology of itraconazole are applied in the field of synthesis of itraconazole, which can solve the problems of poor quality and low yield of crude itraconazole, and achieve the goals of increased yield, high yield and reduced cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 140 g of water, 45 g of sodium hydroxide, 180 g of toluene, 10 g of hydroxyl, 12 g of methylsulfonate, 0.1 g of hydrazine hydrate, and 1.0 g of TEBA into the reaction flask, and heat up to 110°C for 8 hours under reflux . After the reaction, the temperature was lowered to 40°C, and the lye was separated. Continue to cool down to room temperature, and filter to obtain 17.2 g of crude crystals of itraconazole. The yield is 95.0%, and the impurity content is 0.60%.
Embodiment 2
[0036] Add 140 g of water, 45 g of sodium hydroxide, 180 g of toluene, 10 g of hydroxyl, 12 g of methylsulfonate, 0.2 g of hydrazine hydrate, and 1.0 g of TEBA into the reaction flask, and heat up to 110°C for reflux for 10 hours . According to the operation of Example 1, 16.8 g of crude crystals of itraconazole were obtained, with a yield of 93.0% and an impurity content of 0.80%.
Embodiment 3
[0038] Add 140 g of water, 47 g of potassium hydroxide, 180 g of toluene, 10 g of hydroxyl, 12 g of methylsulfonate, 0.1 g of hydrazine hydrate, and 1.0 g of TEBA into the reaction flask, and heat up to 110°C for reflux reaction 7 Hour. According to the operation of Example 1, 17.1 g of crude crystals of itraconazole were obtained, with a yield of 95.0% and an impurity content of 0.62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com