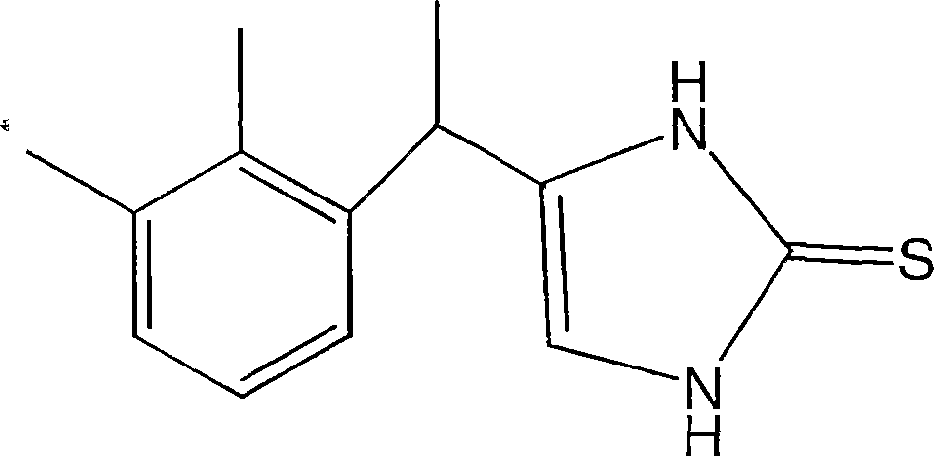
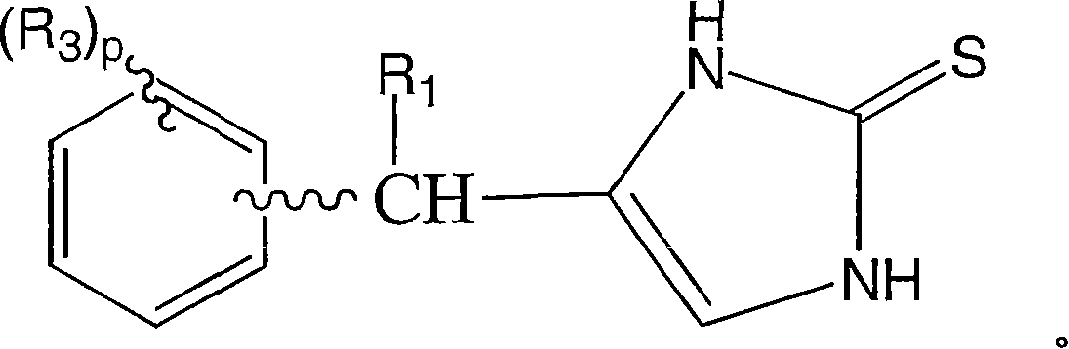
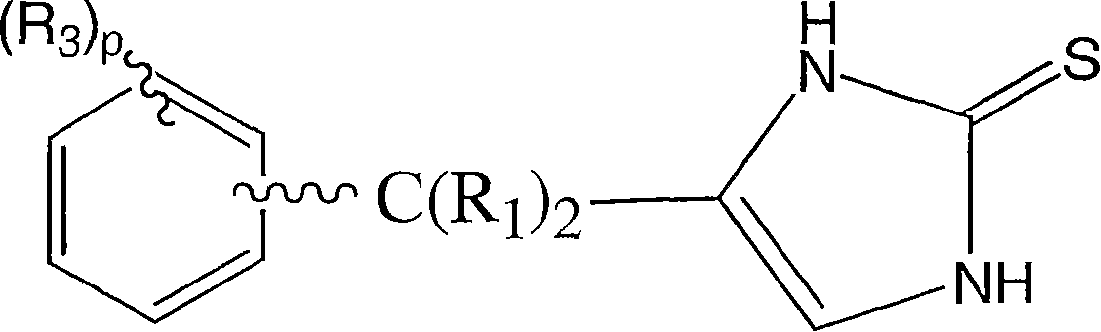4-(phenylmethyl and substituted phenylmethyl)-imidazole-2-thiones acting as specific alpha2 adrenergic agonists
A carbon alkyl, CH2 technology, applied in the field of 4--imidazole-2-thione, can solve the problems of adverse side effects, low blood pressure, inability to provide activity and specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0116] Method A: Method for the preparation of 4-[1-(2,3-dichloro-phenyl)-ethyl]-1,3-dihydro-imidazole-2-thione
[0117] (compound 1)
[0118]
[0119] 4-iodo-1-trityl imidazole (see Cliff et.al. Synthesis (1994) 681, incorporated herein by reference) in dichloromethane (44 mL) at room temperature (rt) (4.4 g, 10.1 mmol ) was treated with ethylmagnesium bromide (3.40 mL, 10.2 mmol, 3M in ether) and allowed to react for 90 minutes. 2-Dichloroacetophenone (from Lancaster) (Intermediate A1 ) (1.0 g, 5.02 mmol) dissolved in dichloromethane (10 mL) was added via syringe at 20 °C and stirred for 16 h. The mixture was quenched with a saturated solution of ammonium chloride (50 mL) and washed with dichloromethane:CH 2 Cl 2 dilution. The organic material was separated by aqueous workup, followed by CH 2 Cl 2 extraction. Chromatography on silica gel with 2% MeOH:CH 2 Cl 2 Purification of the residue afforded 3.8 g of 1-(2,3-dichloro-phenyl)-1-(1-trityl-1H-imidazol-4-yl)-etha...
Embodiment A-2
[0123] Embodiment A-2 (compound 2)
[0124] 2-Fluoroacetophenone (purchased from Lancaster) was used in method A, where 10% Pd / C catalyst was used in step 4 instead of PtO 2 , 4-[1-(2,3-Difluoro-phenyl)-ethyl]-1,3-dihydro-imidazole-2-thione (compound 2) was produced. 1 H NMR (300MHz, methanol-d 4 ): δ7.19-7.06(m, 2H), 6.98-6.92(m, 1H), 6.64(d, J=1.2Hz, 1H), 4.33(q, J=7.2Hz, 1H), 1.55(d, J=7.5Hz, 3H).
Embodiment B
[0126] Method B: Process for the preparation of 4-[1-(2-fluoro-3-trifluoromethyl-phenyl)-ethyl]-1,3-dihydro-imidazole-2-thione (compound 3)
[0127]
[0128] 2-Fluoro-3-trifluoromethylacetophenone (Intermediate B1) (purchased from Lancaster) was used in the appropriate process step of Method A (shown in the reaction scheme above) to obtain 1-(2-fluoro -3-Trifluoromethyl-phenyl)-1-(1-trityl-1H-imidazol-4-yl)-ethanol (intermediate B2).
[0129] Dissolve 1-(2-fluoro-3-trifluoromethyl-phenyl)-1-(1-trityl-1H-imidazol-4-yl) in acetic acid (13 mL) and water (3 mL) - Ethanol (Intermediate B2) (4.7 mmol) was heated to 100° C. for 1 h. The mixture was cooled to room temperature and basified with 2M NaOH. The compound was extracted with ethyl acetate and the solution was concentrated on silica gel. The product was treated with 3-5% NH 3 -MeOH:CH 2 Cl 2 Elution afforded 1-(2-fluoro-3-trifluoromethyl-phenyl)-1-(1H-imidazol-4-yl)-ethanol (intermediate B3) 1.1 g (85%).
[0130] Usi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com