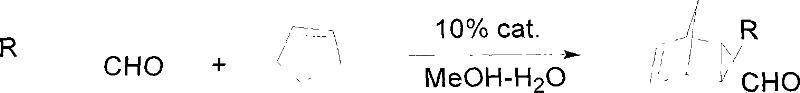Preparing method and application of chiral imidazolinone fungicide decorated by peptide loded with silica gel
An imidazolinone, amino acid technology, applied in the preparation of carbon-based compounds, the preparation of organic compounds, catalysts for physical/chemical processes, etc. Enantioselectivity, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
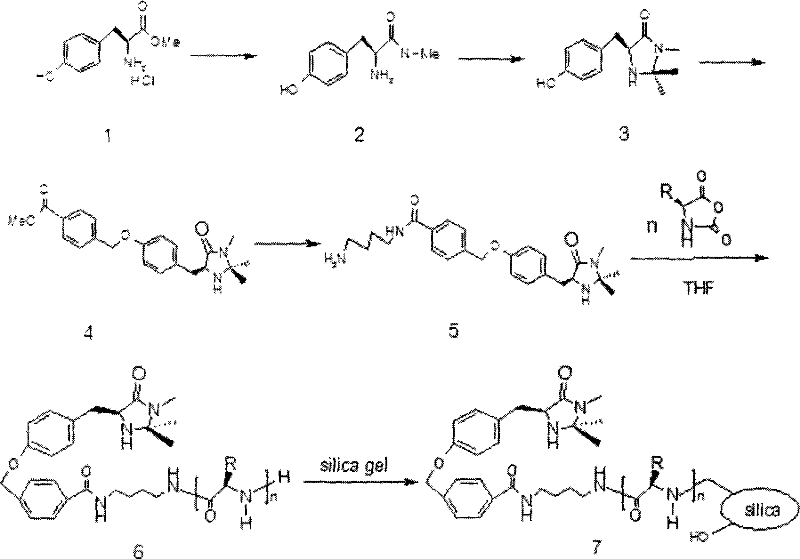
[0029] Example 1: Preparation of a chiral imidazolinone modified with pentameric amino acid loaded on silica gel, the chiral imidazolinone modified with pentameric amino acid loaded on silica gel is the chiral imidazolinone kind of.
[0030] In the first step, 180 g of 25% aqueous solution of methylamine was added to 50 g of raw material 1 tyrosine methyl ester hydrochloride to obtain 47 g of white solid crude product 2, (S)-N-methyl-2-amino-3 -(4-(hydroxyl)phenyl)-propionamide; in the second step, add 60g of acetone, 90g of methanol, and 0.5g of p-toluenesulfonic acid to the crude product 2 to obtain the crude product 3 chiral imidazolidinone , productive rate 50%; In the 3rd step, add the acetone of 90g, the K of 18g successively to crude product 3 2 CO 3, 35g of methyl p-bromomethylbenzoate, to obtain intermediate product 4, (s)-methyl-4((4-((1,2,2-trimethyl-5-oxoimidazolidine-4-alkane Base) methyl)-phenoxy) methyl) benzoate, productive rate 50%; In the 4th step, get the...
Embodiment 2
[0031] Example 2: Preparation of a chiral imidazolinone catalyst modified with octameric leuco amino acid loaded on silica gel, the chiral imidazolinone modified with octameric amino acid loaded on silica gel is a chiral imidazolinone modified with oligomeric amino acid loaded on silica gel One of.
[0032] In the first step, 180 g of 25% aqueous solution of methylamine was added to 50 g of raw material 1 tyrosine methyl ester hydrochloride to obtain 47 g of white solid crude product 2, (S)-N-methyl-2-amino-3 -(4-(hydroxyl)phenyl)-propionamide; in the second step, add 60g of acetone, 90g of methanol, and 0.5g of p-toluenesulfonic acid to the crude product 2 to obtain the crude product 3 chiral imidazolidinone , productive rate 50%; In the 3rd step, add the acetone of 90g, the K of 18g successively to crude product 3 2 CO 3 , 35g of methyl p-bromomethylbenzoate, to obtain intermediate product 4, (s)-methyl-4((4-((1,2,2-trimethyl-5-oxoimidazolidine-4-alkane Base) methyl)-phen...
Embodiment 3
[0033] Example 3: Preparation of a chiral imidazolinone catalyst modified by silica gel-loaded decamerine amino acid, the chiral imidazolinone modified by silica gel-loaded decamerine amino acid is a chiral imidazolinone modified by silica gel-loaded oligomeric amino acid One of.
[0034] In the first step, to 5.0 g of raw material 1 tyrosine methyl ester hydrochloride, add 18 g of methylamine 25% aqueous solution to obtain 4.8 g of white solid crude product 2, (S)-N-methyl-2-amino -3-(4-(hydroxyl) phenyl)-propionamide; Conversion rate 100%; In the second step, add the acetone of 6.0g, the methyl alcohol of 9.0g, the p-toluenesulfonic acid of 0.5g in the crude product 2, Get crude product 3 chiral imidazolinones, productive rate 55%; In the 3rd step, add the acetone of 18g, the K of 3.6g successively to crude product 3 2 CO 3 , 7.0g of methyl p-bromomethylbenzoate to obtain intermediate product 4: (s)-methyl-4((4-((1,2,2-trimethyl-5-oximidazolidine-4- Alkyl) methyl)-phenoxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com