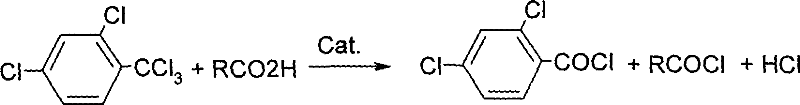Method for synthesizing 2,4-Dichlorobenzoyl chloride
A technology of dichlorobenzoyl chloride and dichlorotrichlorotoluene, which is applied in the field of intermediate synthesis of compounds, can solve the problems of harsh working environment, unstable product quality, and not pleasant enough, and achieve high space-time yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add raw material 2,4-dichlorobenzoic acid 529g (2.0mol), 2,4-dichlorobenzoic acid 382 (2mol), toluene 500ml, catalyst aluminum trichloride 0.13g (0.001mol) into the reactor, adjust The temperature was raised to 120°C, and the reaction was kept for 240 minutes. Sampling analysis: the yield of 2,4-dichlorobenzoyl chloride was 98.8%. Then distill and reclaim toluene, and then collect the finished product of 2,4-dichlorobenzoyl chloride by distillation under reduced pressure; the catalyst aluminum trichloride is recovered from the still residue for the next feeding.
[0022] Result: bp.122-124°C / 15mm, 2,4-dichlorobenzoyl chloride, colorless to pale yellow liquid, GC content 99.22%.
Embodiment 2
[0024] Add 529g (2.0mol) of raw material 2,4-dichlorobenzotrichloride and 0.98g (0.01mol) of catalyst phosphoric acid into the reactor, adjust the temperature to 60°C, add 108g (1.80mol) of acetic acid dropwise, and maintain the temperature of the reactor The temperature was 60°C, and the dropwise addition was completed in about 120 minutes. After the dropwise addition of acetic acid, the insulation reaction was continued for 60 min and the previous fraction was collected. 2,4-Dichlorotrichlorotoluene reacts rapidly with acetic acid under the action of catalyst phosphoric acid to generate acetyl chloride, which is separated from the reactor as the first fraction from the top of the rectifying tower.
[0025] Sampling analysis: the yield of 2,4-dichlorobenzoyl chloride was 90.3%. Then the finished product of 2,4-dichlorobenzoyl chloride was collected by distillation under reduced pressure, which was normal boiling.
[0026] result:
[0027] Front fraction: 50-55℃, acetyl chl...
Embodiment 3
[0030] Add 529g (2.0mol) of the raw material 2,4-dichlorobenzotrichloride and 1.62g (0.01mol) of the catalyst iron trichloride into the reactor, adjust the temperature to 120°C, add 151g (2.04mol) of propionic acid dropwise, and maintain The temperature of the reaction kettle is 120-130°C, and the dropwise addition is completed in about 120 minutes. 2,4-dichlorotrichlorotoluene and propionic acid react rapidly under the action of catalyst ferric chloride to generate propionyl chloride, which is separated from the reactor from the top of the rectifying tower as the first component. After the dropwise addition of propionic acid, the insulation reaction was continued for 210 min. Sampling analysis: the yield of o-chlorobenzoyl chloride is 98.6%. Then, the crude product of propionyl chloride is distilled under reduced pressure in the rectifying tower as the middle distillate, and the crude product of 2,4-dichlorobenzoyl chloride is in the still; the 2,4-dichlorobenzoyl chloride f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com