2,3,4,5-tetrasubstituted derivatives of benzyl ethylene class, preparation method and application
A technology of four-substitution and derivatives, which is applied in the field of 2,3,4,5-tetrasubstituted phenylpropene derivatives and their preparation and application, and can solve the problems of limited drug universal applicability, low selectivity, and ineffective curative effect. Satisfactory etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
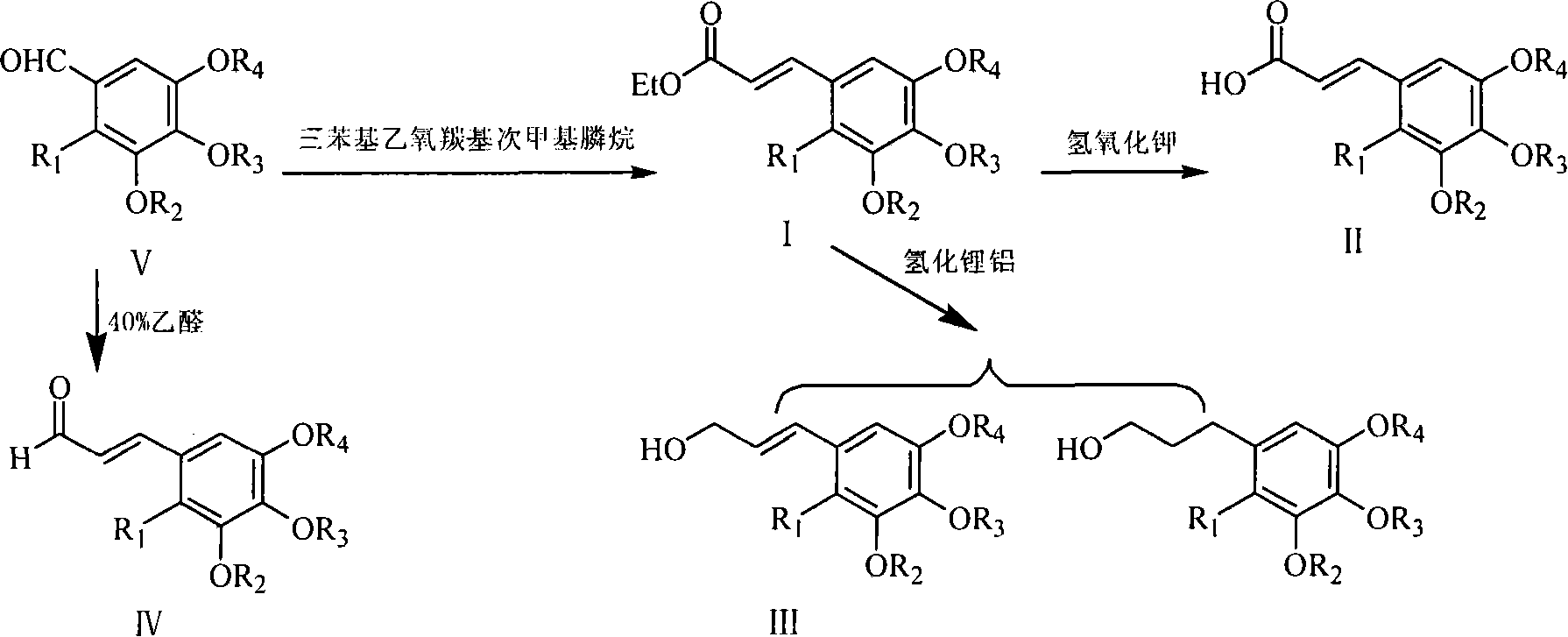
[0077] Embodiment 1: the preparation of V-c (2-bromo-3,5-dimethoxy-4-(3,4-dichlorobenzyloxy) benzaldehyde)
[0078]
[0079] This example relates to a class of 2,3,4,5-tetrasubstituted phenylpropene derivative key intermediate 2,3,4,5-tetrasubstituted benzene with cytotoxic activity shown in formula (V) General synthesis method of formaldehyde series compounds. It specifically relates to the synthesis of compound 2-bromo-3,5-dimethoxy-4-(3,4-dichlorobenzyloxy)benzaldehyde. The compound 2-bromo-4-hydroxyl-3,5-dimethoxybenzaldehyde (0.85 g, 3.28 mmol) was dissolved in 20 ml of acetone, anhydrous potassium carbonate (1.13 g, 8.22 mmol) was added, and stirred for 10 minutes , and then added 3,4-dichlorobenzyl bromide (0.6 ml, 3.61 mmol) in 10 ml of acetone, and refluxed for 4 hours. Thin-layer chromatography (TLC) showed that the reaction of the raw material was basically complete, cooled to room temperature, filtered to remove potassium carbonate, and distilled off the solve...
Embodiment 2
[0081] Embodiment 2: the preparation of compound V-a (2,3,5-trimethoxy-4-(4-ethoxybenzyloxy) benzaldehyde)
[0082]
[0083] This example relates to a class of 2,3,4,5-tetrasubstituted phenylpropene derivative key intermediate 2,3,4,5-tetrasubstituted benzene with cytotoxic activity shown in formula (V) General synthesis method of formaldehyde series compounds. It specifically relates to the synthesis of compound 2,3,5-trimethoxy-4-(4-ethoxybenzyloxy)benzaldehyde. Add p-ethoxybenzyl bromide (0.51 g, 2.37 mmol) sodium iodide (59 mg, 0.40 mmol) and acetone (15 ml) successively in a 100 ml three-necked flask, then add potassium carbonate (0.82 g, 5.94 mmol) and 4-hydroxy-2,3,5-trimethoxybenzaldehyde (420 mg, 1.98 mmol), refluxed for 3 hours. Cool to room temperature, filter, concentrate the filtrate, and purify by column chromatography (petroleum ether / ethyl acetate=15:1, crude product / silica gel=1:30) to obtain 363 mg of white solid with a yield of 53%.
[0084] Compound V...
Embodiment 3-6
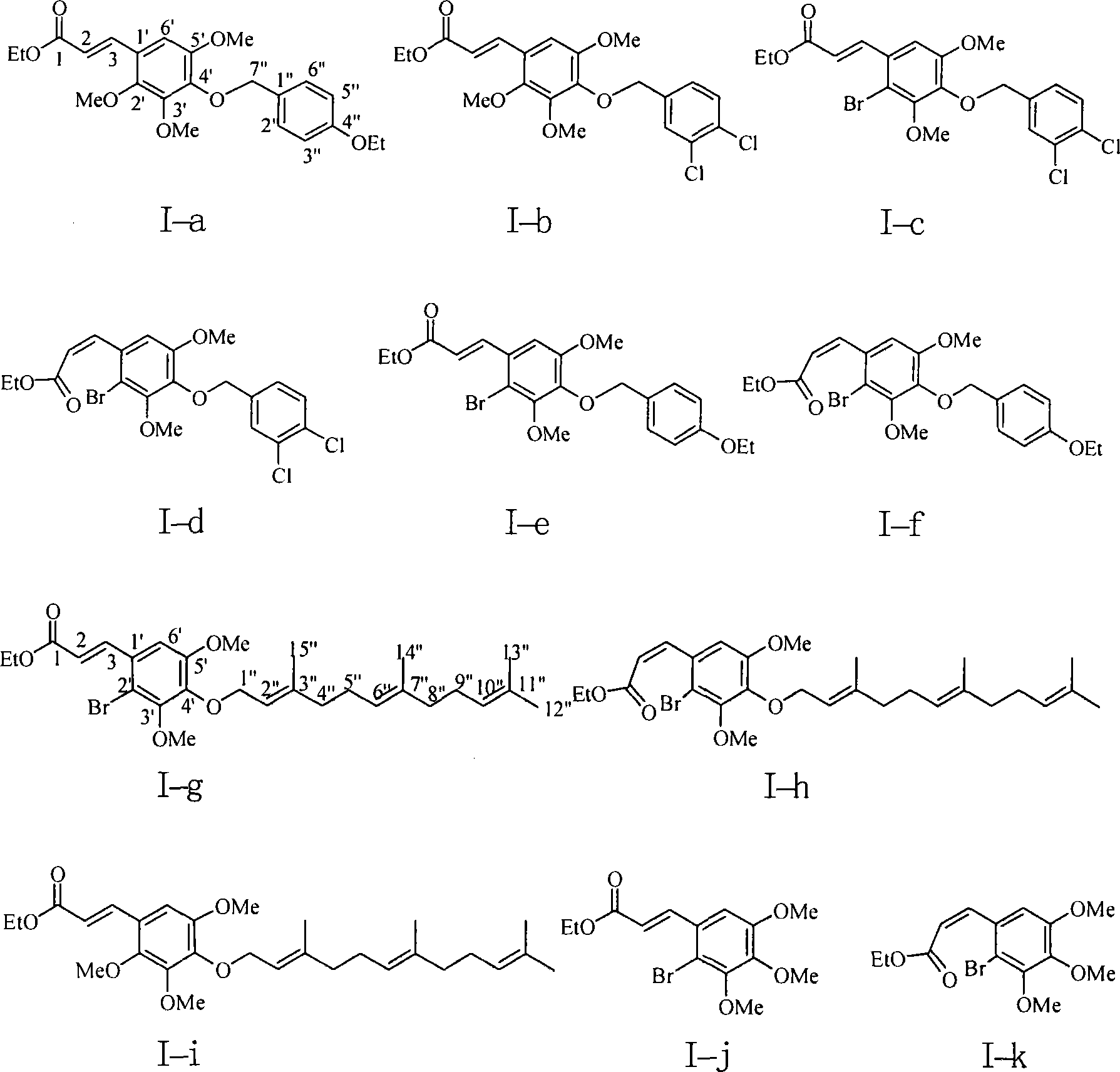
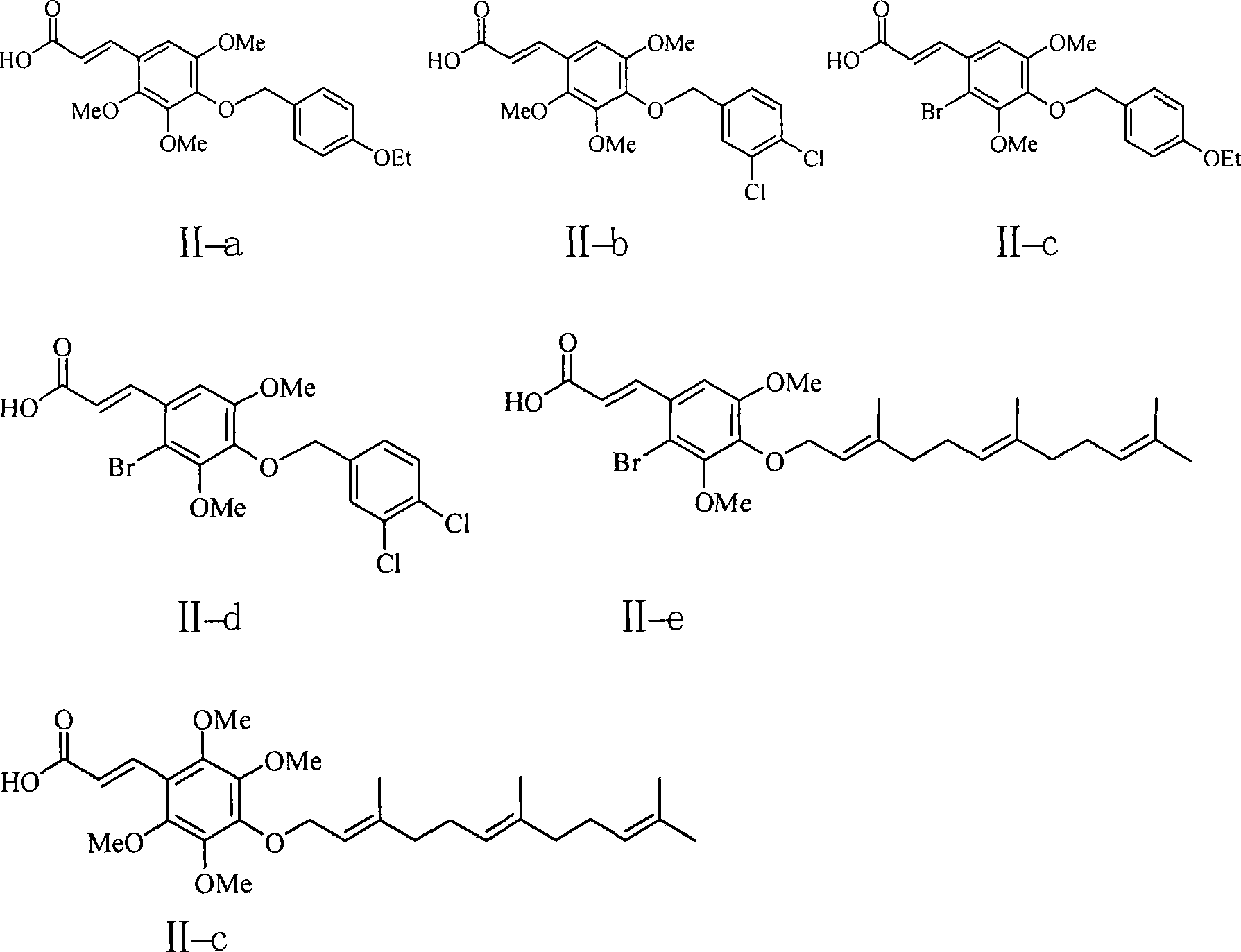
[0085] According to the method with one of embodiment 1-2, prepare embodiment 3-6 compound shown in following table 1:
[0086]
[0087] Table I
[0088]
[0089] List the physicochemical data of each compound in Table 1 below:
[0090] Compound V-b: white solid, Rf (n-hexane / ethyl acetate: 5 / 2) 0.57; 1 H-NMR (400MHz, CDCl 3 ): δ10.29 (1H, s, CHO), 7.62 (1H, d, J=2.0Hz, H-2′), 7.43 (1H, d, J=8.0Hz, H-5′), 7.29 (1H , m, J=8.0, 2.0Hz, H-6'), 7.11 (1H, s, H-6), 5.11 (2H, s, H-7'), 3.93 (3H, s, OCH 3 -2), 3.86 (6H, s, OCH 3 -3, 5).
[0091] Compound V-d: white solid, Rf (n-hexane / ethyl acetate: 5 / 2) 0.51; 1 H-NMR (400MHz, CDCl 3 ): δ10.26 (1H, s, CHO), 7.34 (2H, d, J=8.8Hz, H-3′, 5′), 7.26 (1H, s, H-6), 6.84 (2H, d, J=8.8Hz, H-2', 6'), 5.05(2H, s, H-7'), 4.00(2H, q, J=6.8Hz, OCH 2 CH 3 -4'), 3.87 (6H, s, OCH 3 -3, 5), 1.38 (3H, t, J=6.8Hz, OCH 2 CH 3 -4′).
[0092] Compound V-e: colorless liquid, Rf (n-hexane / ethyl acetate: 5 / 2) 0.61; 1 H-NMR (400MHz, CDCl3): δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com