Hydroxyphenyl cross-linked macromolecular network and applications thereof
A technology of macromolecules and hydroxybenzene, which is applied in the cross-linked macromolecular network of hydroxybenzene and its application fields, and can solve the problems of unstable results and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
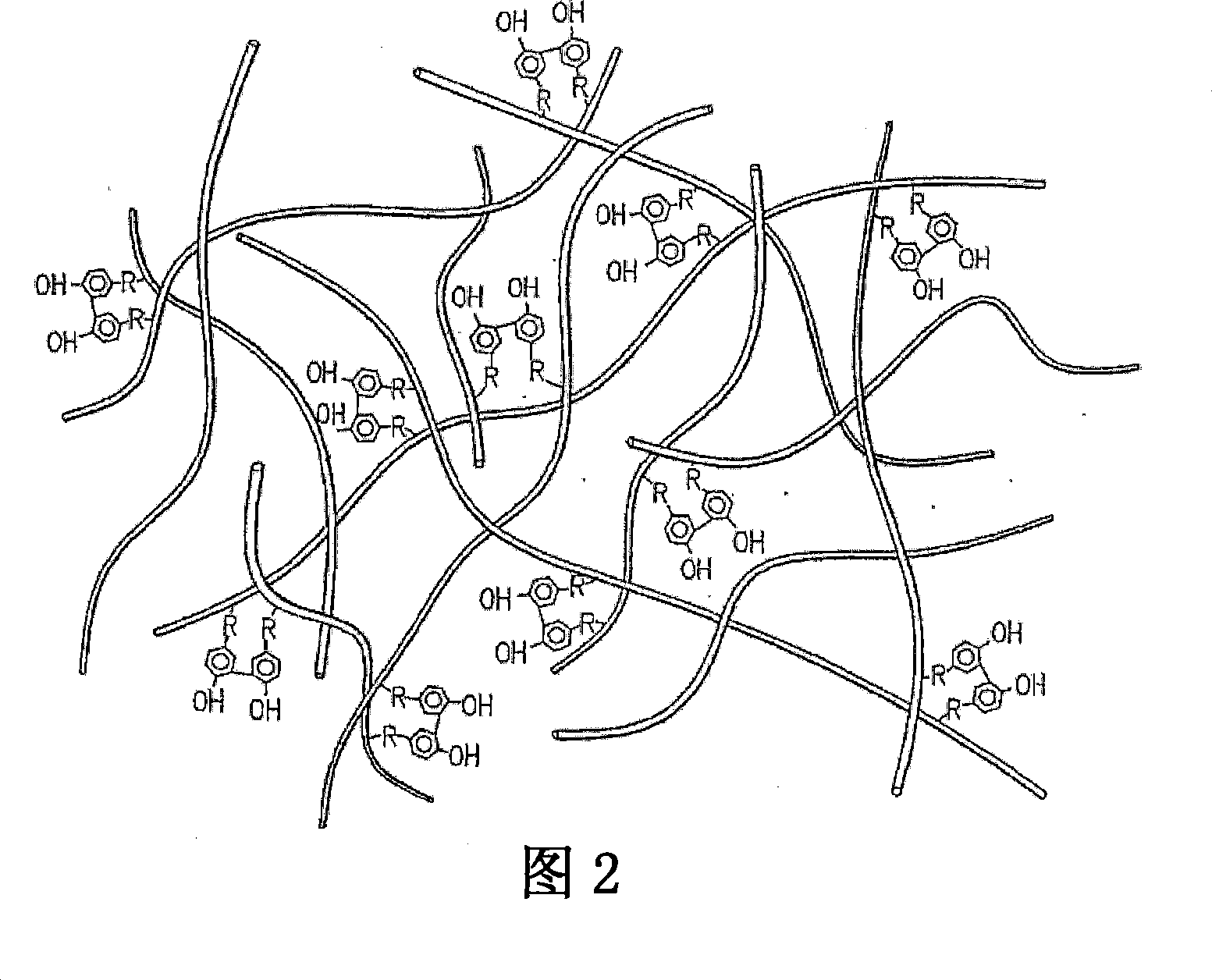
[0161] The experimental dosage of the tyramine-substituted hyaluronic acid hydrogel with dityramine crosslinking of the present invention was prepared according to the following method. HA was dissolved in 250 mM 2-(N-morpholine)ethanesulfonic acid (MES), 150 mM NaCl, 75 mM NaOH, pH 6.5 at a concentration of 1 mg / ml based on hexuronic acid, and the solution contained relative to HA carboxyl groups The molar concentration of the groups is 10-fold excess of tyramide. Tyramide substitution on the starting carboxyl groups of EDC was subsequently added in a 10-fold excess relative to the molar concentration of HA carboxyl groups. N-hydroxysuccinimide (NHS) was added to the reaction at a molar ratio of 1 / 10 relative to the molar amount of EDC to assist the EDC-catalyzed amidation reaction by forming an activated ester. The reaction was carried out at room temperature for 24 hours, followed by extensive dialysis against 150 mM NaCl followed by ultrapure water followed by lyophilizat...
Embodiment 2
[0163] Experiments were performed to determine the extent of tyramide substitution (and subsequent dityramide crosslinking) of the T-HA macromolecular network of the present invention. First, 3 formulations of (uncrosslinked) tyramide-substituted hyaluronic acid (T-HA) were prepared according to the method described above, named OX, 1X or 10X. OX formulations were prepared without EDC (i.e. no carbodiimides), meaning no carbodiimides were present to mediate tyramine NH 2 group and HA molecule CO 2 A reaction between H groups to form an amide bond. Therefore, the OX formulation can be considered as a control. The 1X formulation contains CO on the HA molecule in the reaction mixture 2 The stoichiometric ratio of the amount of H groups is 1:1 for EDC. The 10X formulation contains CO on the HA molecule in the reaction mixture 2 The stoichiometric ratio of the amount of H groups is 10:1 (or 10-fold excess) of EDC. In all three formulations, the stoichiometric excess of tyrami...
Embodiment 3
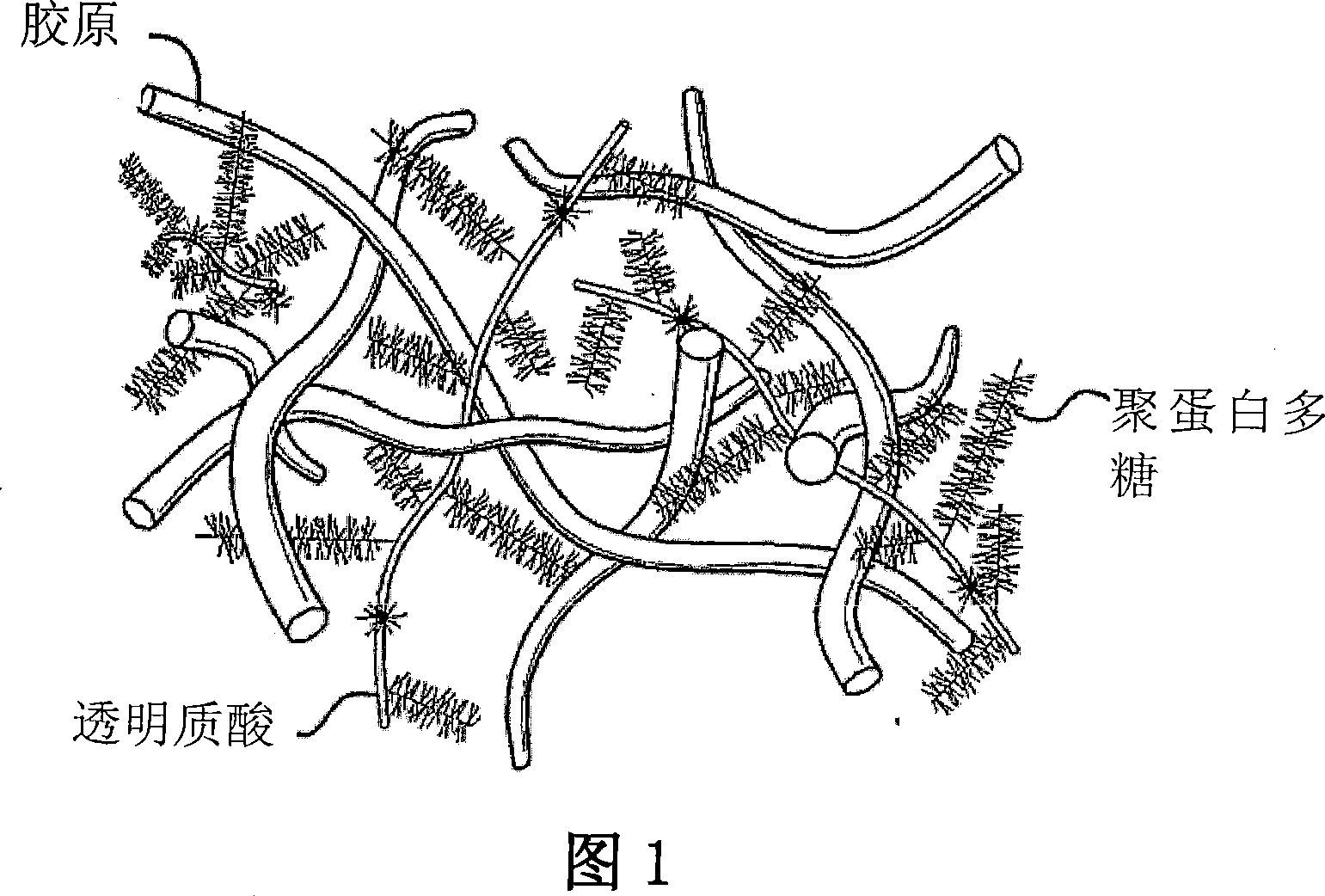
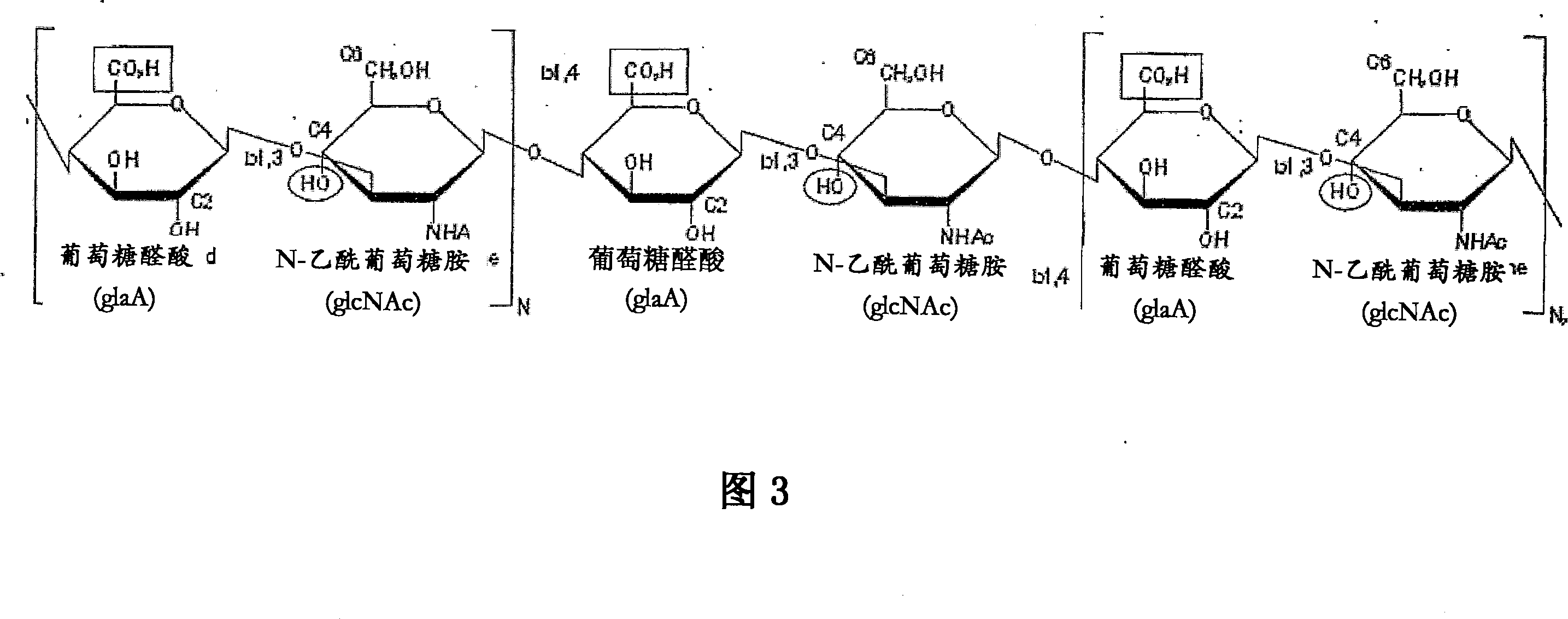
[0170] Traditionally, it has been believed that natural cartilage is viscoelastic and is mainly due to the presence of negatively charged SO on adjacent chondroitin sulfate chains in the aggrecan matrix. 4 2- Due to the repulsive forces between the groups, natural cartilage is able to resist deformation and absorb compressive loads. Tests are carried out to determine that the macromolecular network that only contains dityramide cross-linked hyaluronic acid molecules (i.e. no aggrecan and chondroitin sulfate) in the present invention has no SO 4 2- Efficacy in resisting deformation and absorbing pressure in the case of cartilage compared with natural cartilage. Specifically, three kinds of networks were prepared respectively: 1) HA molecules cross-linked by dityramine (T-HA); 2) chondroitin sulfate in the form of proteoglycans cross-linked by dityramide (T-Aggrecan); and 3) A composite material composed of 50% T-HA and 50% T-Aggrecan. An uncrosslinked T-HA formulation with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com