Protein allergens derivative
A technology of allergens and derivatives, applied in the fields of peptide/protein components, allergic diseases, plant peptides, etc., can solve the problem of IgE binding ability reduction and achieve the effect of scaling up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0293] Example 1: Characterization of Hypoallergenic Derivatives from Timothy Grass Pollen Arrestin
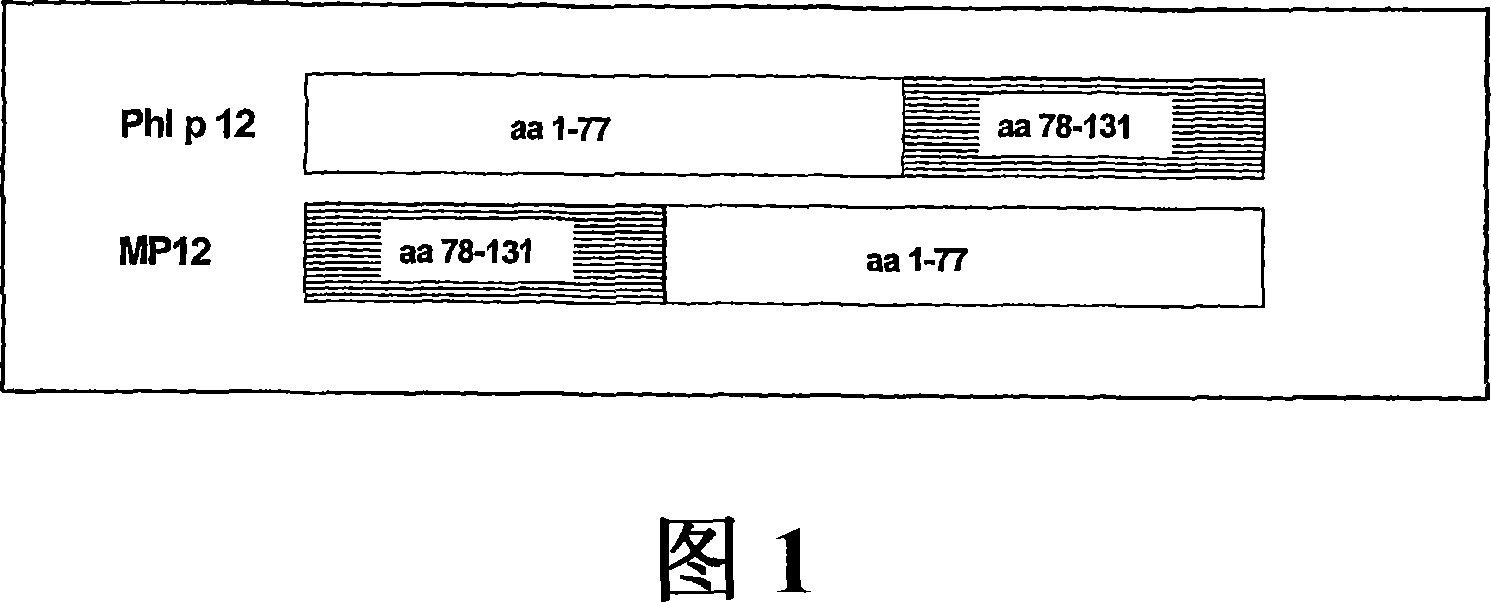
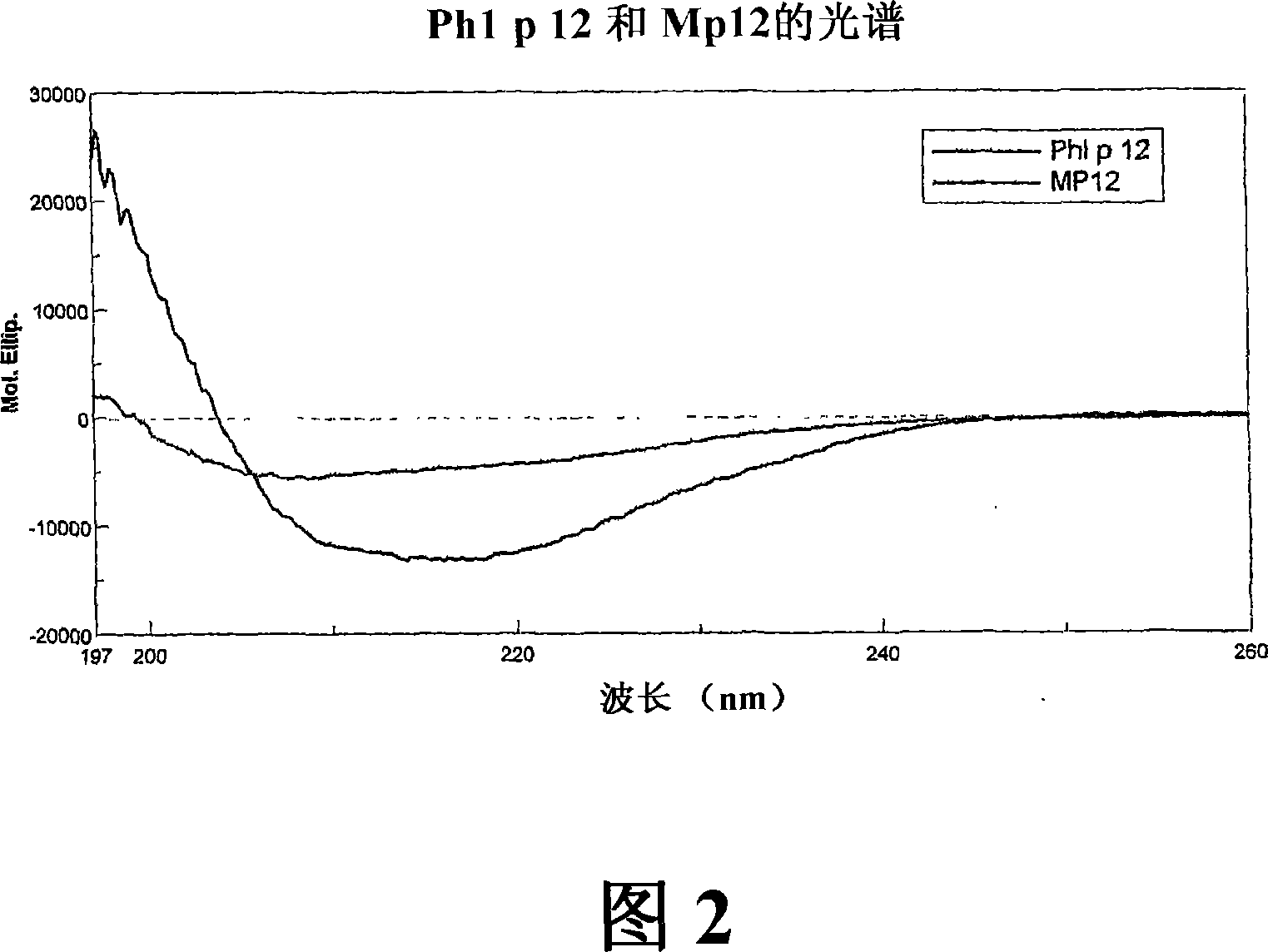
[0294] a) Generation, expression and purification of Phl p 12, a hypoallergenic variant of Timothy grass pollen suppressor protein
[0295] Genetic engineering of recombinant Phl p 12-derivatives using overlap PCR technique. The PCR template was the cDNA encoding the Timothy grass pollen suppressor protein, Phl p 12, subcloned in the pet17b expression vector. The following primers were used to generate two PCR fragments for protein purification containing overlapping sequences as well as NdeI and EcoRI restriction sites and sequences encoding the carboxy-terminal 6x Histidin residues. For fragment 1, primer MDE-1: 5'CATATGAGGCCCGGCGCGGTCATC3' and primer MDE-2: 5'GTACGTCTGCCACGCCATCATGCCTTGTTCAAC3' were used, and for fragment 2, primer MABC-1: 5'GTTGAACAAGGCATGATGTCGTGGCAGACG3' and primer MABC-2: 5'GAATTCTTAATGGTGATGGTGATGGTGACCCTGTAG were used. In the next step, both PCR pro...
Embodiment 2
[0305] Example 2: Reduction of IgE binding ability of MP12
[0306] a) MP12 exhibits significantly reduced IgE binding capacity
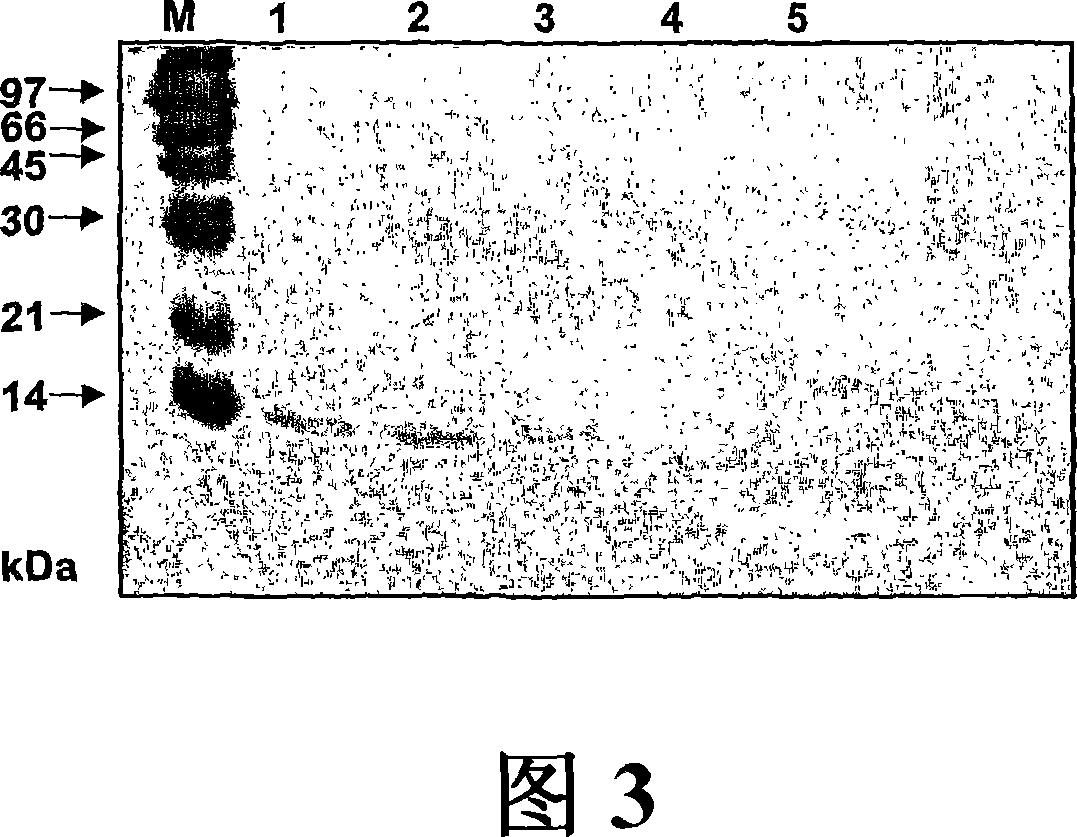
[0307] The IgE binding ability of recombinant MP12 was compared with recombinant Phl p 12 wild type by dot analysis using sera from 24 arrestin-sensitized patients ( FIG. 4 ). Phl p 12 and MP12 and human serum albumin (HSA) for control were spotted onto nitrocellulose and probed with sera from 24 arrestin-sensitized patients. use 125 I-labeled anti-human-IgE antibody detects bound IgE antibody. All patients showed IgE reactivity with PhI p 12 wild type, while none of the 24 patients reacted with MP12 or with the control protein HSA (Figure 4).
[0308] To quantify the reduction in the IgE binding capacity of MP12, fluid phase inhibition was performed. For this, sera from 6 arrestin-sensitized patients were preincubated with 10 μg of Phl p 12 or with MP12 and subsequently incubated with ELISA plate-bound Phl p 12 (5 μg / ml). Bound IgE antibodies ...
Embodiment 3
[0315] Example 3: Immunization with MP12 induces IgG antibodies that recognize Phl p 12 wild type as well as arrestins from other pollens.
[0316] To test whether immunization with the shuffled Phl p 12 induces IgG antibodies reactive with Phl p 12 wild-type and arrestin from other pollen, Freund's complete and incomplete adjuvant (200 μg / injection) (Charles River, Kisslegg, Germany), rabbits were immunized three times with Phl p 12 or MP12. Serum samples were obtained at four week intervals. Serum was stored at -20°C until analysis.
[0317]The reactivity of IgG antibodies induced by MP12 and Phl p 12 was analyzed by ELISA (Fig. 6). Phl p 12 and arrestins from birch (Bet v 2) and mugwort were spread on ELISA plates (5 μg / ml) and incubated with serial dilutions (1:2000-1:64000) of rabbit antisera . Bound rabbit antibodies were detected with peroxidase-labeled donkey anti-rabbit antiserum (AmershamPharmacia Biotech) diluted 1:1000.
[0318] MP12 induced an IgG anti-Phl p ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com