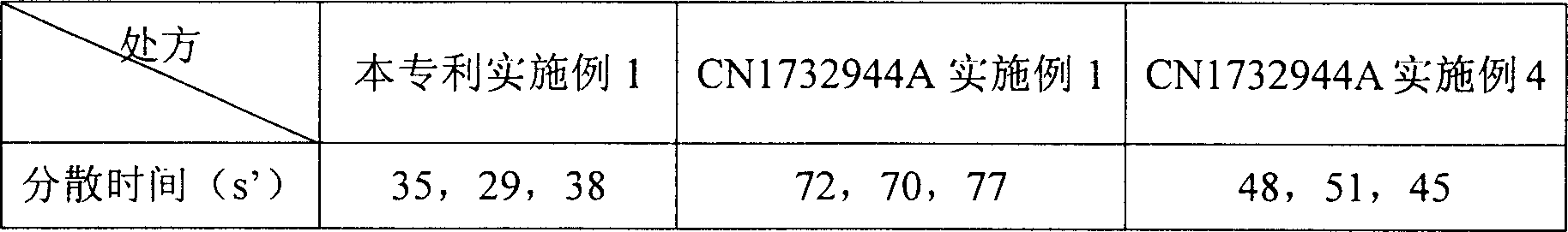
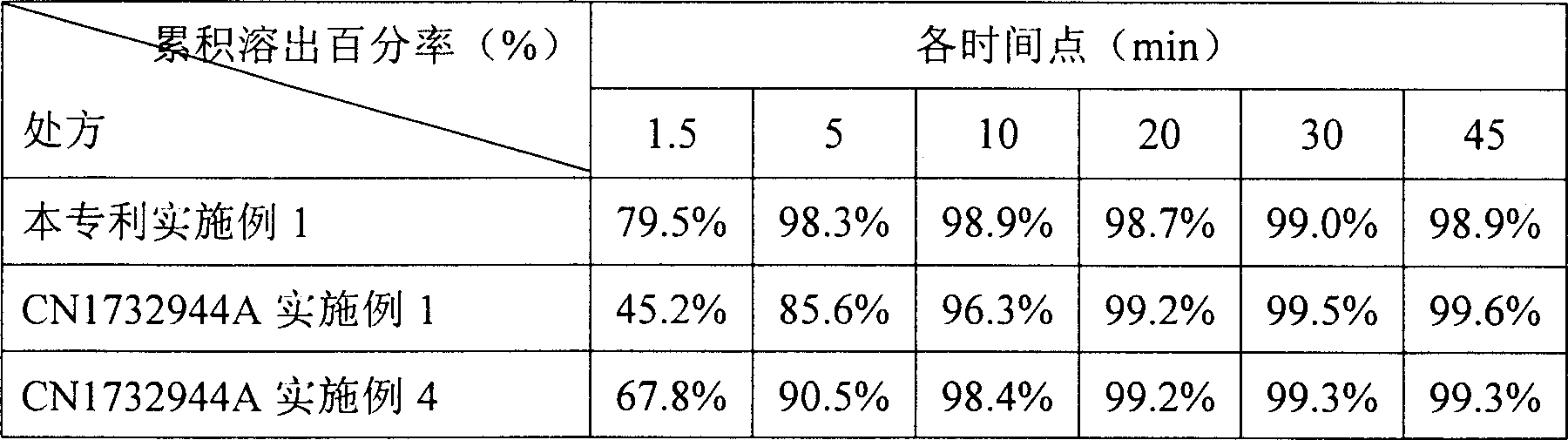
Entecavir dispersing tablet and preparing method
A technology of entecavir and dispersible tablets, which is applied in the field of entecavir dispersible tablet preparations and its preparation, can solve the problems of entecavir dispersible tablets with poor taste, unreasonable selection of binders, and poor taste, so as to improve bioavailability and reduce adverse reactions , improve the effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Prescription: Entecavir 0.5g
[0032] Mannitol 40g
[0033] Lactose 60g
[0034] Microcrystalline Cellulose 60g
[0035] Low-substituted hydroxypropyl cellulose (internal addition) 12.6g
[0036] Crospovidone (extra) 9g
[0037] Micronized silica gel 3.6g
[0038] Magnesium stearate 1.8g
[0039] 20% ethanol solution of hypromellose
[0040] A total of 1000 pieces were made
[0041] Preparation method: first crush the above materials through a 100-mesh sieve, fully mix entecavir, mannitol, lactose, microcrystalline cellulose, and low-substituted hydroxypropyl cellulose, and then add dilute alcohol solution of hypromellose to prepare Soft material, granulate with 20 mesh sieve, dry at 60°C for 1.5-2 hours, granulate with 18 mesh sieve, add disintegrant crospovidone, lubricant micronized silica gel, magnesium stearate, mix well, measure content, adjust tablet weight and make entecavir dispersible table...
Embodiment 2
[0043] Prescription: Entecavir 1.5g
[0044] Mannitol 35g
[0045] Lactose 70g
[0046] Microcrystalline Cellulose 55g
[0047] Low-substituted hydroxypropyl cellulose (internal addition) 14g
[0048] Crospovidone (additional) 8g
[0049] Micronized silica gel 3.7g
[0050] 40% ethanol solution of hypromellose
[0051] A total of 1000 pieces were made
[0052] Preparation method: first crush the above materials through a 100-mesh sieve, fully mix entecavir, mannitol, lactose, microcrystalline cellulose, and low-substituted hydroxypropyl cellulose, and then add hypromellose in 40% ethanol solution Make soft material, granulate with 20-mesh sieve, dry at 60°C for 1.5-2 hours, granulate with 18-mesh sieve, add disintegrating agent cross-linked povidone and lubricant micro-powder silica gel, measure the content after mixing, and adjust the tablet weight , compressed into entecavir dispersible tablets.
Embodiment 3
[0054] Prescription: Entecavir 0.5g
[0055] Xylitol 30g
[0056] 70g pregelatinized starch
[0057] Microcrystalline Cellulose 60g
[0058] Low-substituted hydroxypropyl cellulose (internal and external) 16.2g
[0059] Micronized silica gel 3.6g
[0060] Magnesium stearate 1.8g
[0061] 20% ethanol solution of hypromellose
[0062] A total of 1000 pieces were made
[0063]Preparation method: first crush the above materials through a 100-mesh sieve, fully mix entecavir, xylitol, pregelatinized starch, microcrystalline cellulose, and low-substituted hydroxypropyl cellulose, and then add hypromellose Soft material made from 20% ethanol solution, granulated with a 20-mesh sieve, dried at 60°C for 1.5-2 hours, granulated with a 18-mesh sieve, and disintegrating agent low-substituted hydroxypropyl cellulose and lubricant micro-powdered silica gel, stearic acid added Magnesium, measure the content after mixing, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com