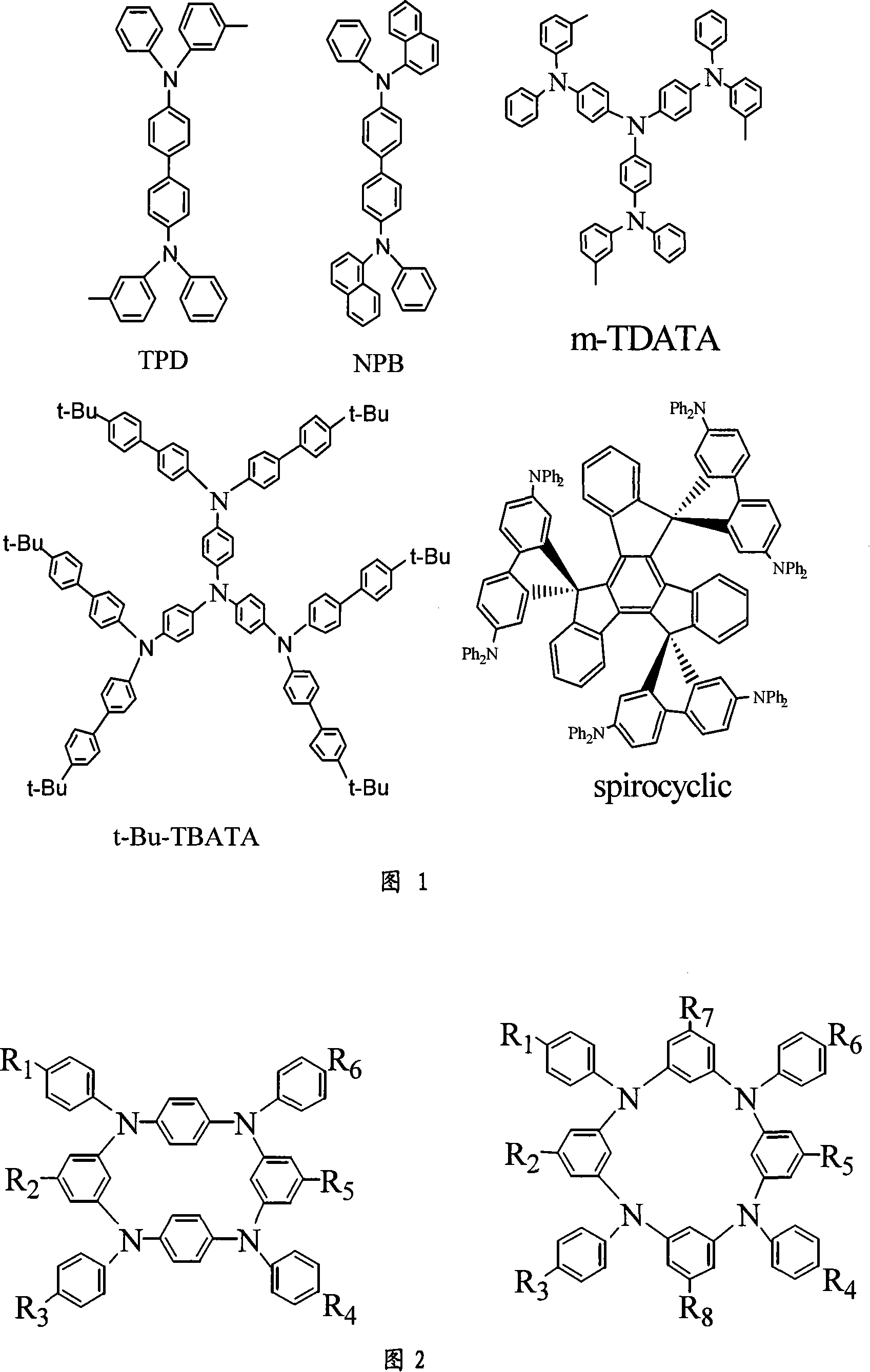
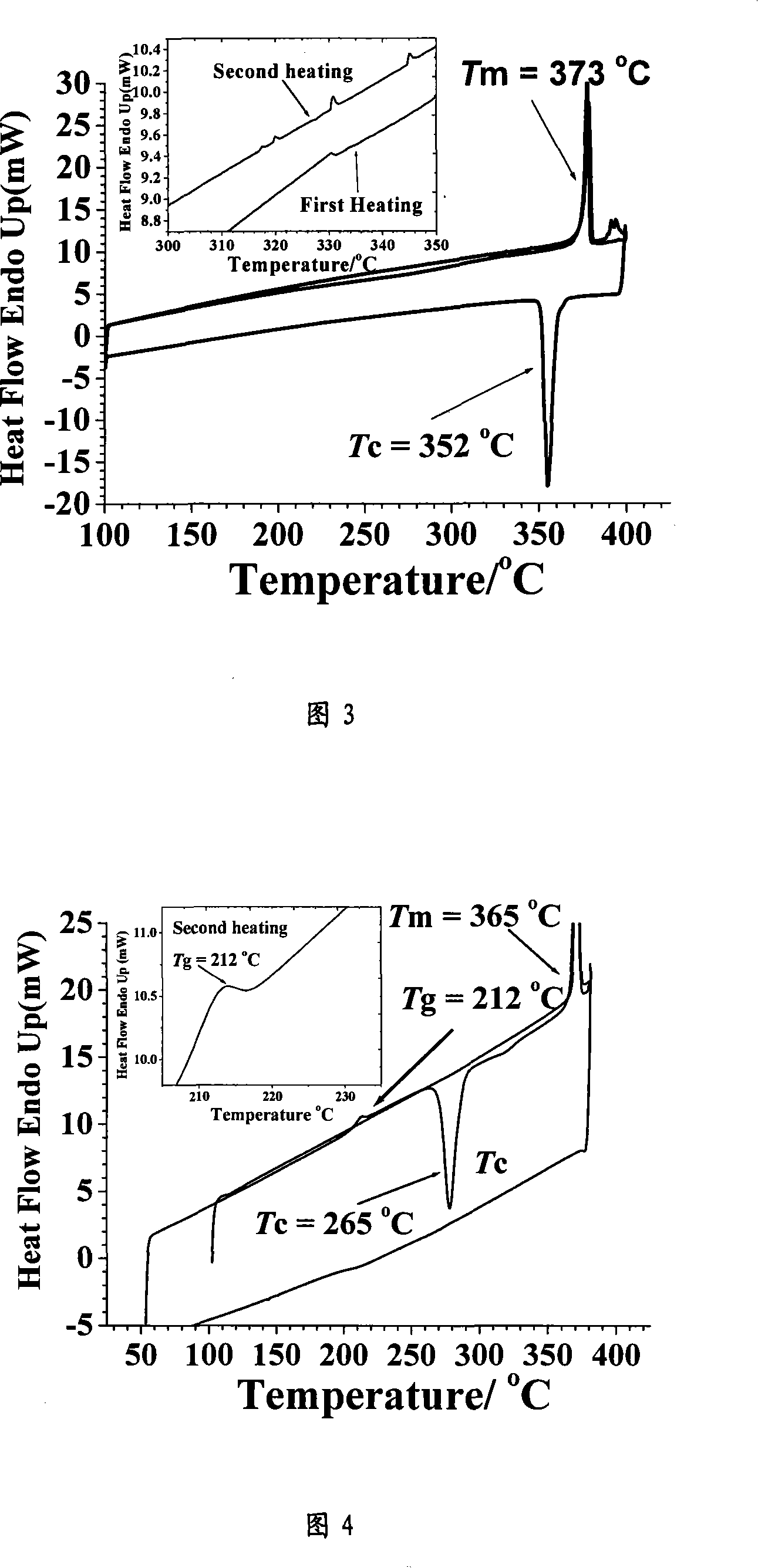
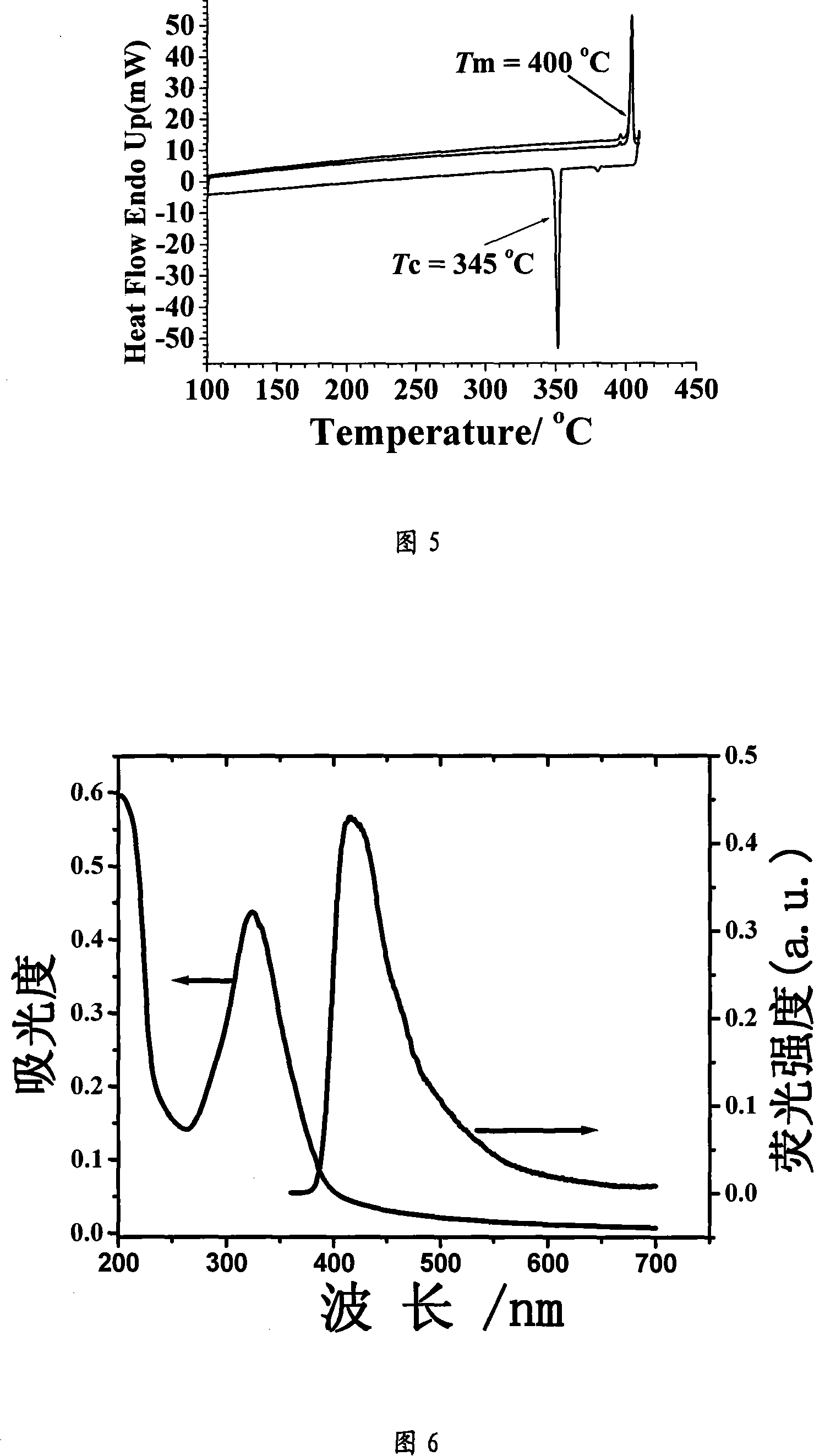
Cyclic arylamine as hole transferring material with high vitrification point and its synthesis process
A hole transport material and high vitrification technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A cyclic aromatic amine compound (abbreviated as CAA), its structural formula is:
[0026]
[0027] Its synthetic method is: in 250 milliliters of three-neck flasks, add 50 milliliters of o-xylenes, add N, N '-diphenyl-p-phenylenediamine (5mmol), m-dibromobenzene (5mmol), sodium tert-butoxide ( 12mmol), then add catalyst palladium acetate (0.02mmol) and tri-tert-butylphosphine (0.025mmol), and reflux at 130°C for 12 hours. After the reaction, the solvent was distilled off under reduced pressure, and the residue was recrystallized from tetrahydrofuran, separated by column chromatography with toluene as the eluent, and then sublimated by gradient to obtain the pure product. NMR analysis 1 H NMR (400MHz, CDCl 3 ), the results are: 7.26-7.20 (m, 18H), 6.70 (s, 6H), 6.86 (s, 7H), 6.67 (s, 3H), 6.33 (s, 2H). The elemental analysis result of this compound (molecular formula is C 48 h 36 N 4 ): Theoretical value: C, 86.20; H, 5.42; N, 8.38. Tested value: C, 86.26; H, 5...
Embodiment 2
[0029] N, N'-diphenyl substituted cyclic aromatic amine compounds, its structural formula is:
[0030]
[0031] Its synthesis method is: add 50 milliliters of o-xylene in 250 milliliters of three-neck bottles, add N, N'-bis(4-N, N'-diphenylphenyl)-p-phenylenediamine (5mmol), N, N- diphenyl substituted m-dibromobenzene (5mmol), sodium tert-butoxide (12mmol), then add catalyst palladium acetate (0.02mmol) and tri-tert-butylphosphine (0.025mmol), and reflux at 130°C for 12 hours. After the reaction, the solvent was distilled off under reduced pressure, and the residue was recrystallized from tetrahydrofuran, separated by column chromatography using toluene as the eluent, and then sublimated by gradient to obtain the pure product. Elemental analysis (molecular formula is C 120 h 90 N 10 ): Theoretical value: C, 86.23; H, 5.39; N, 8.38. Tested value: C, 86.25; H, 5.37; N, 8.42.
Embodiment 3
[0033] Its structural formula is:
[0034]
[0035] Its synthetic method is: in 250 milliliters three-neck flasks, add 50 milliliters of o-xylenes, add N, N '-diphenyl-m-phenylenediamine (5mmol), m-dibromobenzene (5mmol), sodium tert-butoxide ( 12mmol), then add catalyst palladium acetate (0.02mmol) and tri-tert-butylphosphine (0.025mmol), and reflux at 130°C for 12 hours. After the reaction, the solvent was distilled off under reduced pressure, and the residue was recrystallized from tetrahydrofuran, separated by column chromatography using toluene as the eluent, and then sublimated by gradient to obtain the pure product.
[0036] The elemental analysis result of this compound (molecular formula is C 48 h 36 N 4): Theoretical value: C, 86.23; H, 5.39; N, 8.38. Tested value: C, 86.58; H, 5.46; N, 8.60.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com