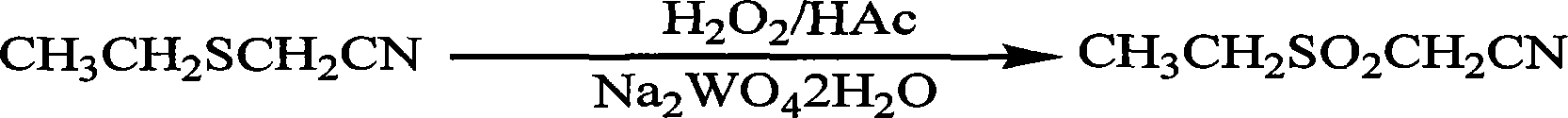Method for preparing ethylsulfonyl acetonitrile
A technology of ethylsulfonylacetonitrile and ethylthioacetonitrile, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problem of high price of oxidant m-chloroperoxybenzoic acid, and achieve mild reaction and high content The effect of high and low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Add 375.0g (3.71mol) of ethylthioacetonitrile, 225.0g (3.75mol) of acetic acid and 18.0g of tungsten dihydrate to a 3000mL three-necked flask equipped with a thermometer, condenser, constant pressure dropping funnel, and stirrer Sodium acid, add 984.0g (7.8mol) hydrogen peroxide (content 27.0%) dropwise at room temperature, stir and react at 40°C to 50°C for 2 hours, then distill off about 750.0g aqueous acetic acid solution under reduced pressure. Cool and add enough water to dissolve the salt. The oil layer was separated, and the water layer was extracted twice with 500 mL of chloroform. The organic layers were combined, and the chloroform was recovered under reduced pressure to obtain 461.9 g of the product, with a crude yield of 93.6% and a content of 98.4% (quantitative analysis by gas chromatography). 1 H NMR (CDCl 3 )δ: (ppm) 1.50 ~ 1.55 (t, 3H, -CH 2 C H 3 ), 3.30~3.37 (q, 2H, -CH 2 -), 3.97~3.98(m, 2H, -CH 2 CN).
Embodiment 2
[0015] Add 375.0g (3.71mol) of ethylthioacetonitrile, 225.0g (3.75mol) of acetic acid and 18.0g of tungsten dihydrate to a 3000mL three-necked flask equipped with a thermometer, condenser, constant pressure dropping funnel, and stirrer Sodium acid, add 984.0g (7.8mol) hydrogen peroxide (content 27.0%) dropwise at room temperature, stir and react at 40°C to 50°C for 2 hours, then distill off about 750.0g aqueous acetic acid solution under reduced pressure. Cool and add enough water to dissolve the salt. The oil layer was separated, and the aqueous layer was extracted twice with 500 mL of ethyl acetate. The organic layers were combined, and ethyl acetate was recovered under reduced pressure to obtain 470.0 g of the product, with a crude yield of 95.2% and a content of 98.6% (quantitative analysis by gas chromatography).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com