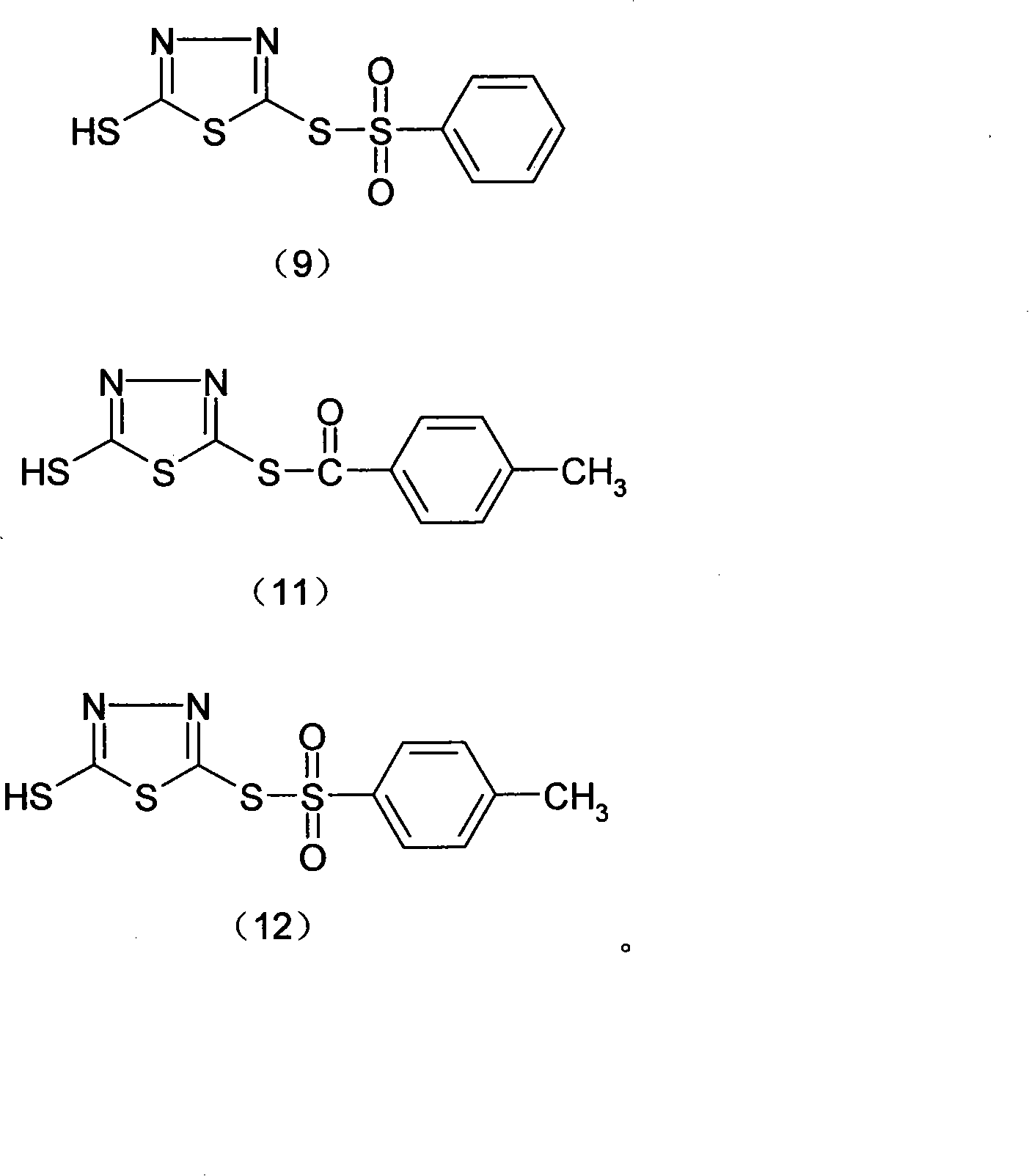
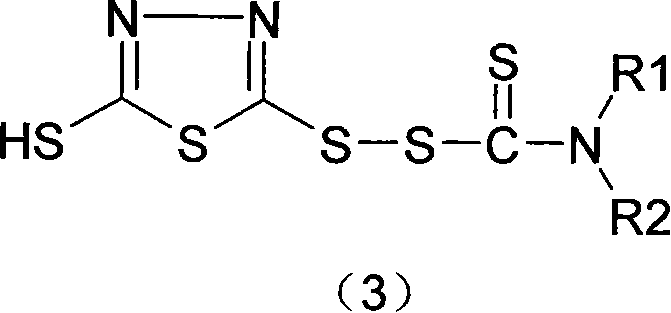
Thiadiazoles derivative for halopolymer vulcanization crosslinking and synthetic method for the same
A technology of thiadiazole derivatives, vulcanization and cross-linking, which is applied in the direction of organic chemistry, can solve the problems of difficult post-processing, poor comprehensive performance of rubber products, and low yield of products, and achieve simplified post-processing operations and excellent mechanical properties. Excellent and simple synthetic method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1: Synthesis process of thiadiazole derivative (8)
[0094]
[0095] (1) Add 20g of NaOH (0.5mol) and 70ml of water to a 1L four-necked flask equipped with a stirrer, thermometer, reflux tube, and constant pressure dropping funnel to make a NaOH aqueous solution, stir and dissolve completely, and then give it to the flask through an ice water bath. The temperature of the solution drops to 7°C to 10°C and becomes a transparent solution. Add 76 g of DMTD (0.5 mol) to 300 ml of ethanol (70%) and stir to dissolve into a yellow, clear and transparent solution. Cool down to 10°C, and then slowly add 76 g of DMTD in ethanol to the flask through a dropping funnel. Stir carefully to diffuse the heat released in time until the solution in the four-neck flask becomes a yellow, clear and transparent solution, and then filter the solution to remove the insoluble matter. (During the process of dissolving DMTD in an alkaline solution, heat is released, and the temperature of the...
Embodiment 2
[0099] Example 2: Synthesis process of thiadiazole crosslinking agent (9)
[0100]
[0101] (1) Add 28g KOH (0.5mol) and 75ml water to a 1L four-necked flask equipped with a stirrer, thermometer, reflux tube, and constant pressure dropping funnel to make a KOH aqueous solution, stir and dissolve through an ice water bath. The temperature of the solution is reduced to about 10°C to become a transparent solution. Add 76 g of DMTD (0.5 mol) to 300 ml of ethanol and stir to dissolve it into a yellow, clear and transparent solution. The temperature is reduced to about 10°C, and then the ethanol solution of 76 g of DMTD dissolved in the flask is slowly added to the flask through a dropping funnel and stirred continuously. The released heat is diffused in time until the solution in the four-necked flask becomes a yellow, clear and transparent solution, and then the solution is filtered to remove the insoluble matter. (During the process of dissolving DMTD in an alkaline solution, heat ...
Embodiment 3
[0105] Example 3; Synthesis process of thiadiazole derivative (10)
[0106]
[0107] (1) Add 20g of NaOH (0.5mol) and 70ml of water to a 1L four-necked flask equipped with a stirrer, thermometer, reflux tube, and constant pressure dropping funnel to make a NaOH aqueous solution, stir and dissolve completely, and then give it to the flask through an ice water bath. The temperature of the solution drops to about 7°C and becomes a transparent solution. Add 76 g of DMTD (0.5 mol) to 300 ml of ethanol and stir to dissolve it into a yellow, clear and transparent solution. The temperature is lowered to 10°C, and then slowly add 76 g of DMTD in ethanol to the flask through a dropping funnel. The released heat diffuses in time until the solution in the four-necked flask becomes yellow, clear and transparent, and then the solution is filtered to remove the insoluble matter. (During the process of dissolving DMTD in an alkaline solution, heat is released, and the temperature of the mixture...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com