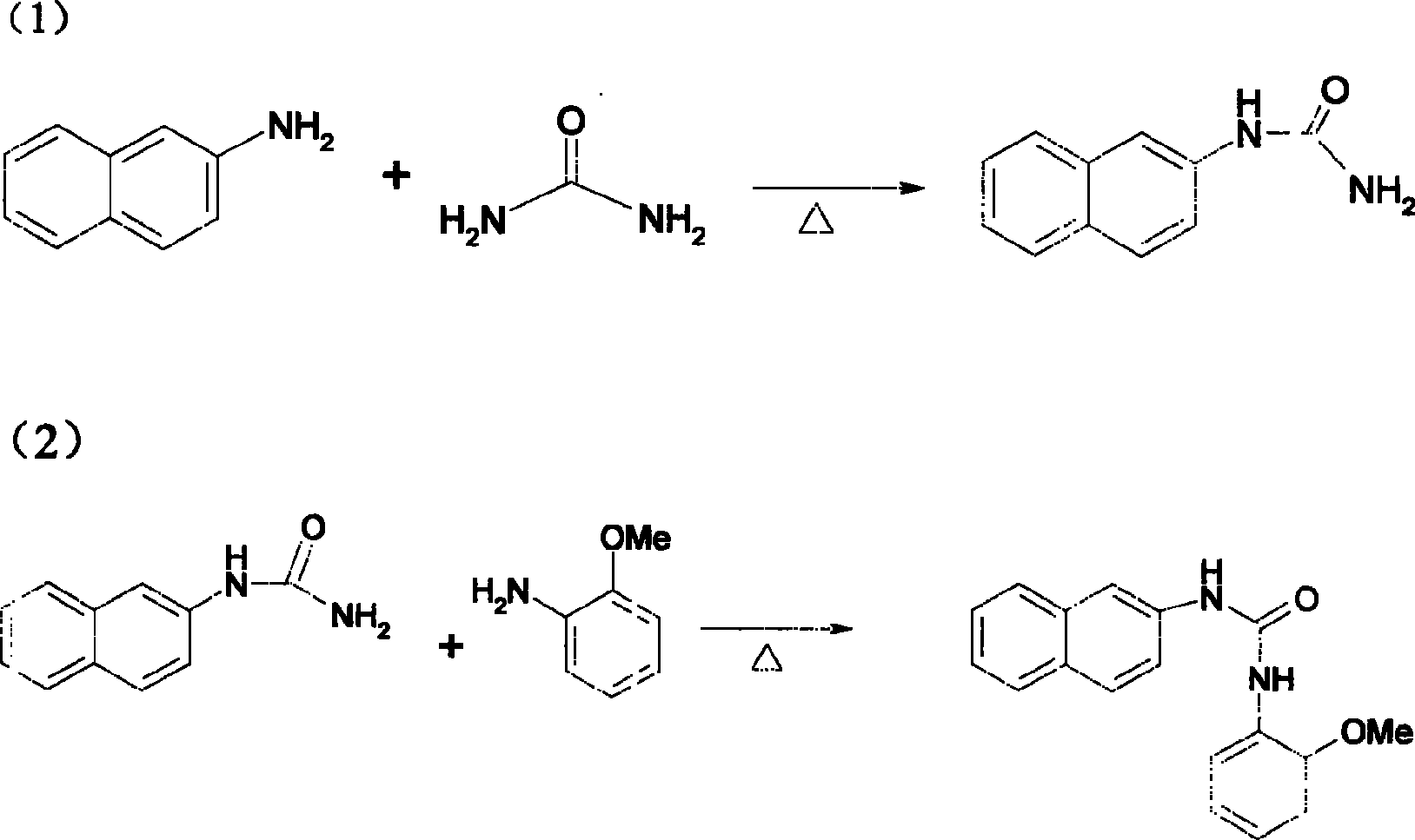
Method of manufacturing 1-(2-methoxyphenyl)-3-naphthyl group-2-urea
A technology of naphthyl and naphthylamine, applied in the direction of amide active ingredients, organic chemistry, antibacterial drugs, etc., can solve the problems of enhanced drug resistance of pathogenic bacteria, reduce drug costs, reduce minimum inhibitory concentration, and solve resistance The effect of the drug on bacterial infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 2
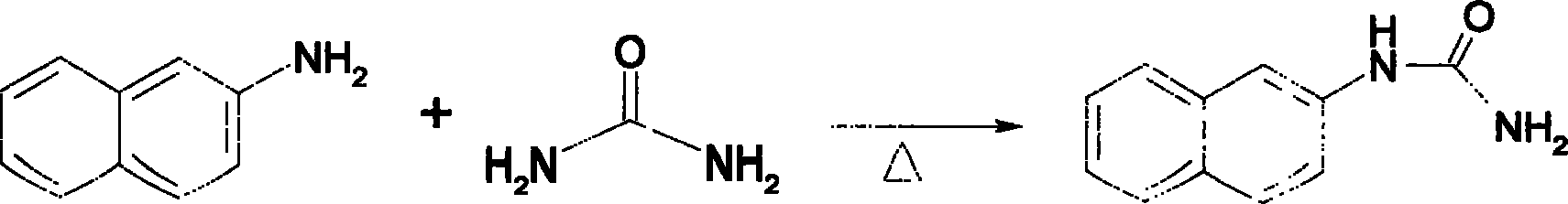
[0018] The reaction process is: (1) 143.2g of β-naphthylamine and 62g of urea were added to 300ml of dilute sulfuric acid, heated to reflux for 2 hours, cooled and filtered to obtain 179g of 1-(naphthalene-2 base)-urea. Yield 96%.
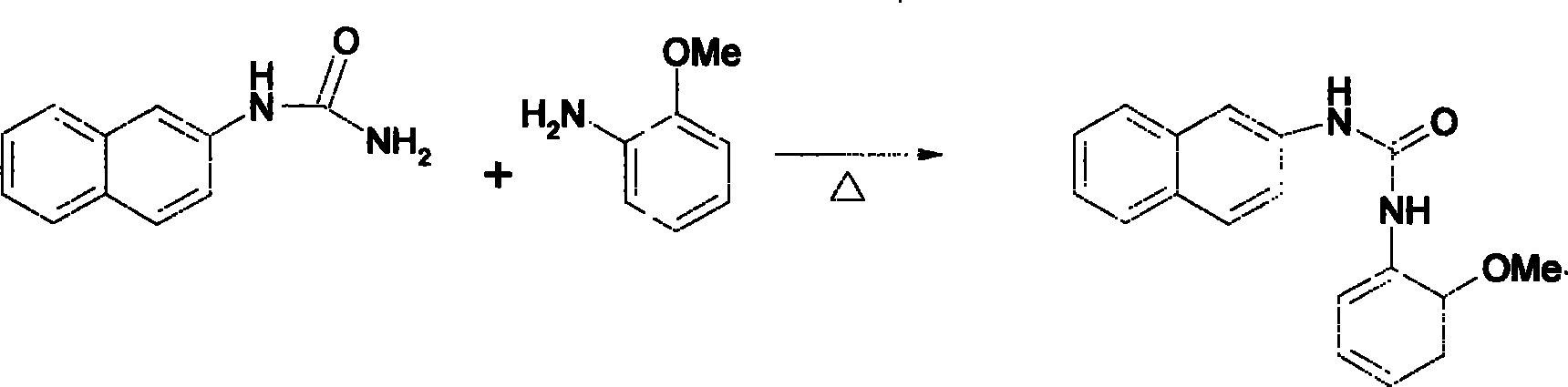
[0019] Reaction (2) 186.2g of 1-(naphthalene-2 base)-urea was mixed with 125g of anisole and 300ml of dilute sulfuric acid, heated to 100°C for 3 hours, cooled, and filtered to obtain 1-(2-methoxy 270 g of phenyl)-3-naphthyl-2-urea. The total yield of the two-step reaction is 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com