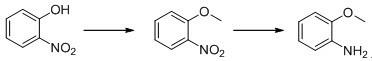
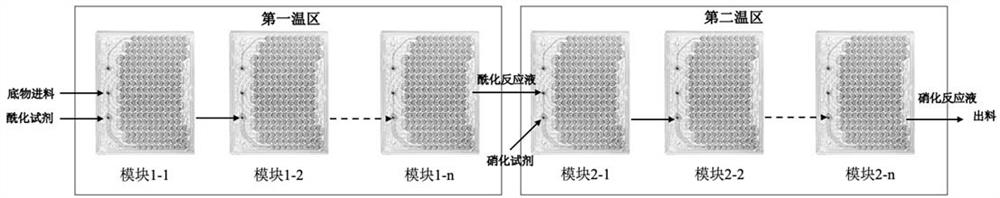
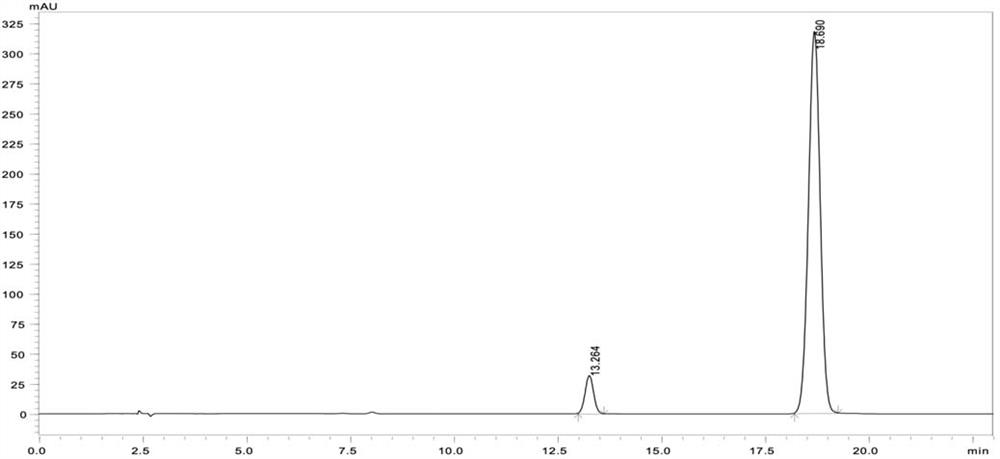
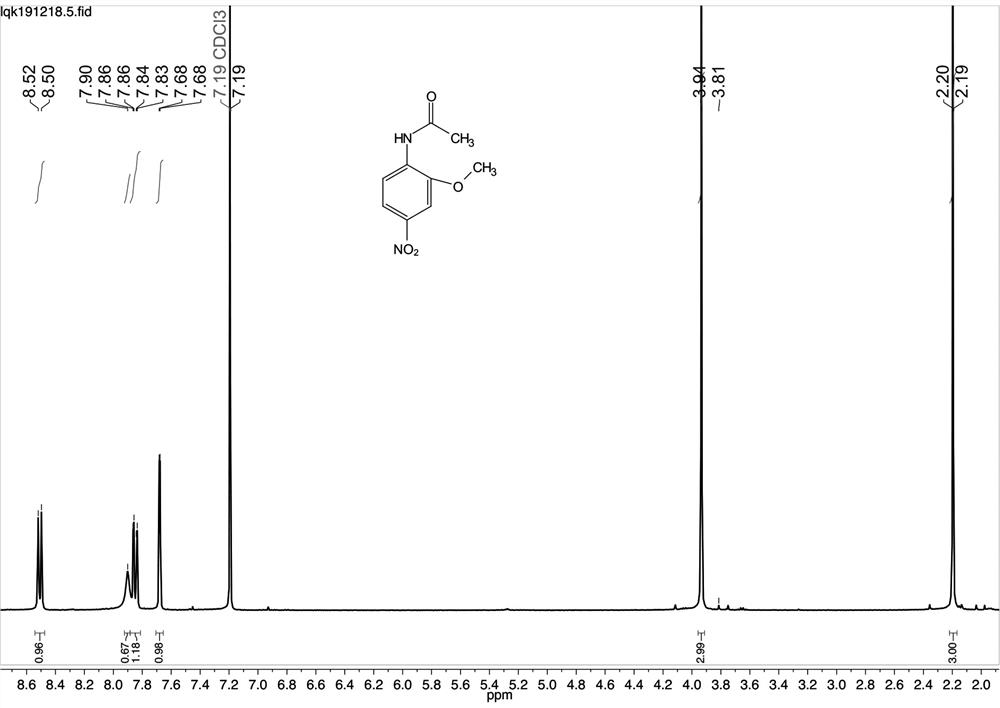
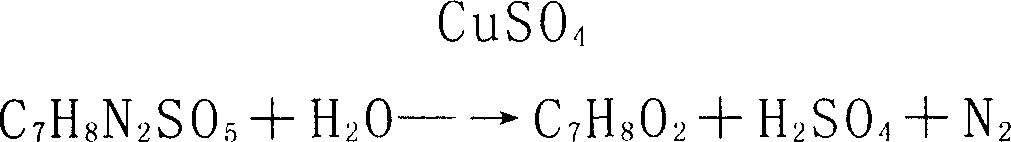Patents
Literature
50 results about "O-Anisidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
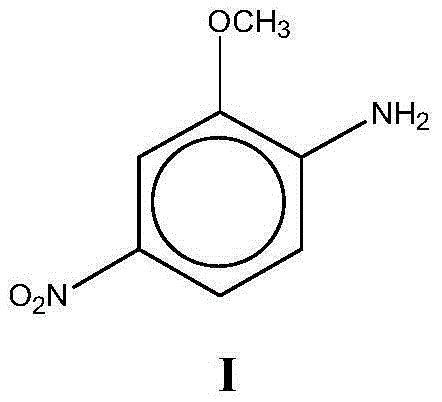
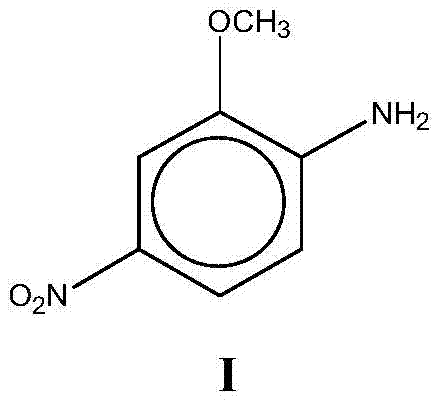
O-Anisidine (2-anisidine) is an organic compound with the formula CH₃OC₆H₄NH₂. A colorless liquid, commercial samples can appear yellow owing to air oxidation. It is one of three isomers of the methoxy-containing aniline derivative.
A kind of production method of anthranil
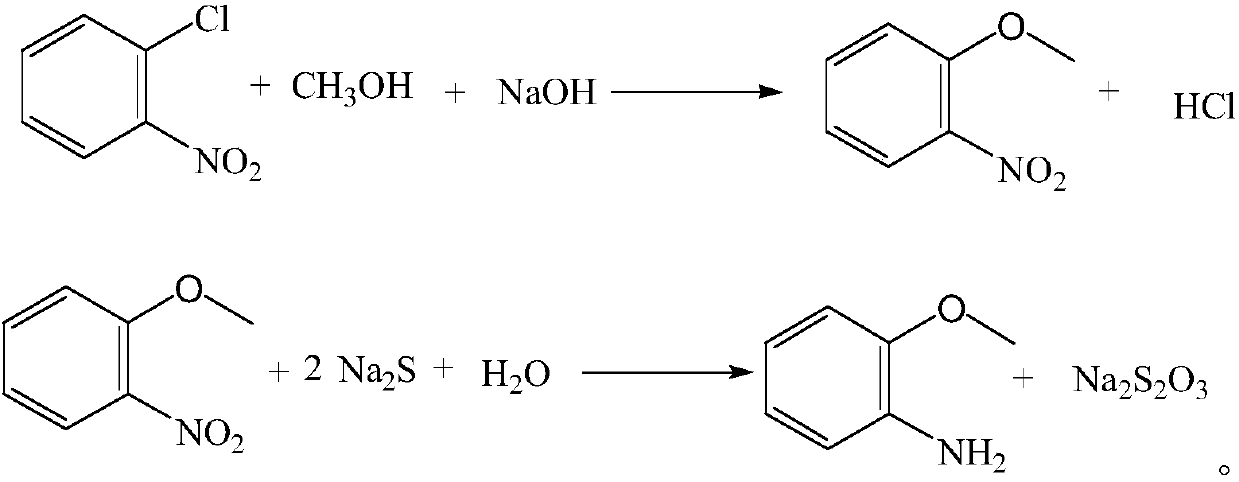
ActiveCN102276483AReduce consumptionSimple processOrganic compound preparationAmino-hyroxy compound preparationO-nitrochlorobenzeneSodium methoxide
The invention provides a production method of oamino pheylmethyl ether, and the method comprises the following steps of: reacting ortho nitrochlorobenzene serving as a raw material with sodium methoxide for carrying out a methoxylation reaction to obtain orthonitroanisole; secondly, carrying out hydrogenation reduction on the orthonitroanisole by using methanol as a solvent in the presence of a catalyst to prepare the oamino pheylmethyl ether; and finally, carrying out dealcoholization, dehydrogenation and refining on reactants to obtain the oamino pheylmethyl ether as a finished product. The production process comprises the following steps of: preparing sodium methoxide, etherifying the ortho nitrochlorobenzene, distilling the methanol and nitroether for separation, hydrogenating the orthonitroanisole, distilling a hydrogenating solution for separating the oamino pheylmethyl ether and treating wastewater. The production method has the characteristics of simple process, short procedure, continuity in reaction, high production efficiency, good product quality, less energy consumption, concentrated purification of reaction wastewater and no emission, and is suitable for producing the oamino pheylmethyl ether by using the ortho nitrochlorobenzene as the raw material.
Owner:LIAONING SHIXING PHARMA & CHEM
Process for preparing aminoanisol and aniline by using mixture of nitroanisole and nitro chlorobenzene as raw materials
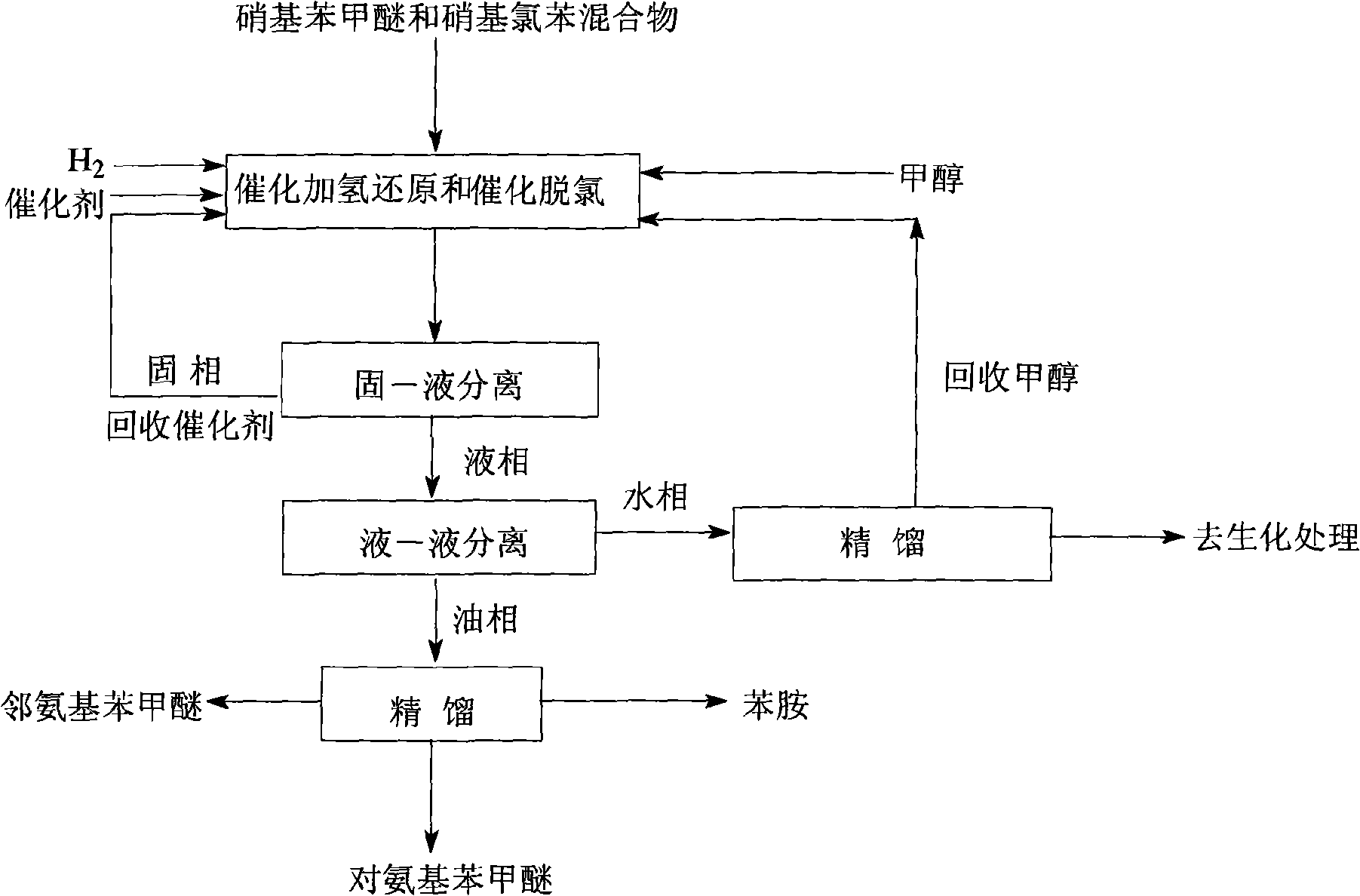
ActiveCN101307000AReduced conversion rate requirementsEasy to separateOrganic compound preparationAmino compound preparationDistillationOil phase
The invention relates to a process for preparing aminoanisole and aniline by taking a mixture of a nitroanisole and nitrochlorobenzene as a raw material. The process comprises the following: (1) a step of the catalytic hydrogenation reaction and the catalytic dechlorination reaction, during which, methanol is taken as a solvent, the mixture of the nitroanisole and the nitrochlorobenzene is taken as the raw material, catalyst is added, hydrogen is aerated; (2) a step of the solid-liquid separation, during which, solid and liquid in the material obtained from the step (1) after the catalytic hydrogenation reaction and the catalytic dechlorination reaction are separated, a liquid phase comprises aminoanisole, aniline, methanol, hydrochloride and water and is utilized in the next step; (3) a step of the liquid-liquid separation, during which, the oil phase-water phase liquid-liquid separation is performed in the material obtained in the step (2), the aminoanisole and aniline in the oil phase is utilized in the next step; (4) a step of distillation separation, during which, the distillation separation is performed in the oil phase to produce products of paraphenetidine, ortho-anisidine and aniline. The process for preparing the aminoanisole and the aniline has the advantages that: (1) the cost is low, the preliminary treatment step is saved, the process is simple; (2) the process is clean, the energy consumption is low, the 'three wastes' are little; (3) the purity of the product is high.
Owner:江苏仁欣化工股份有限公司
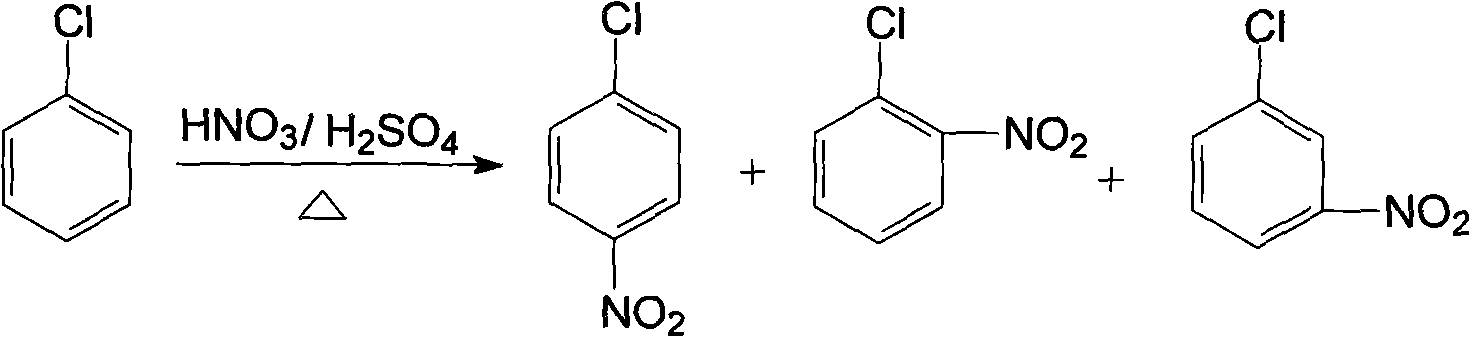
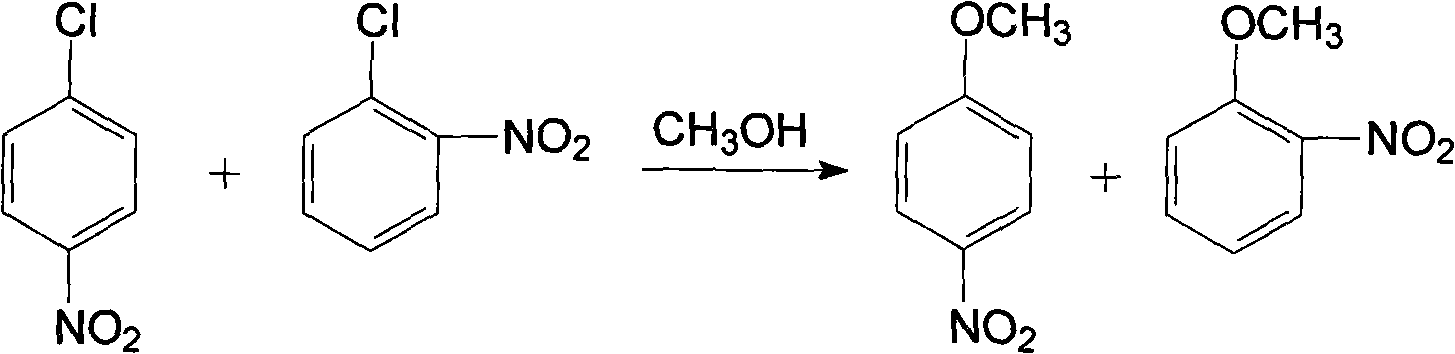
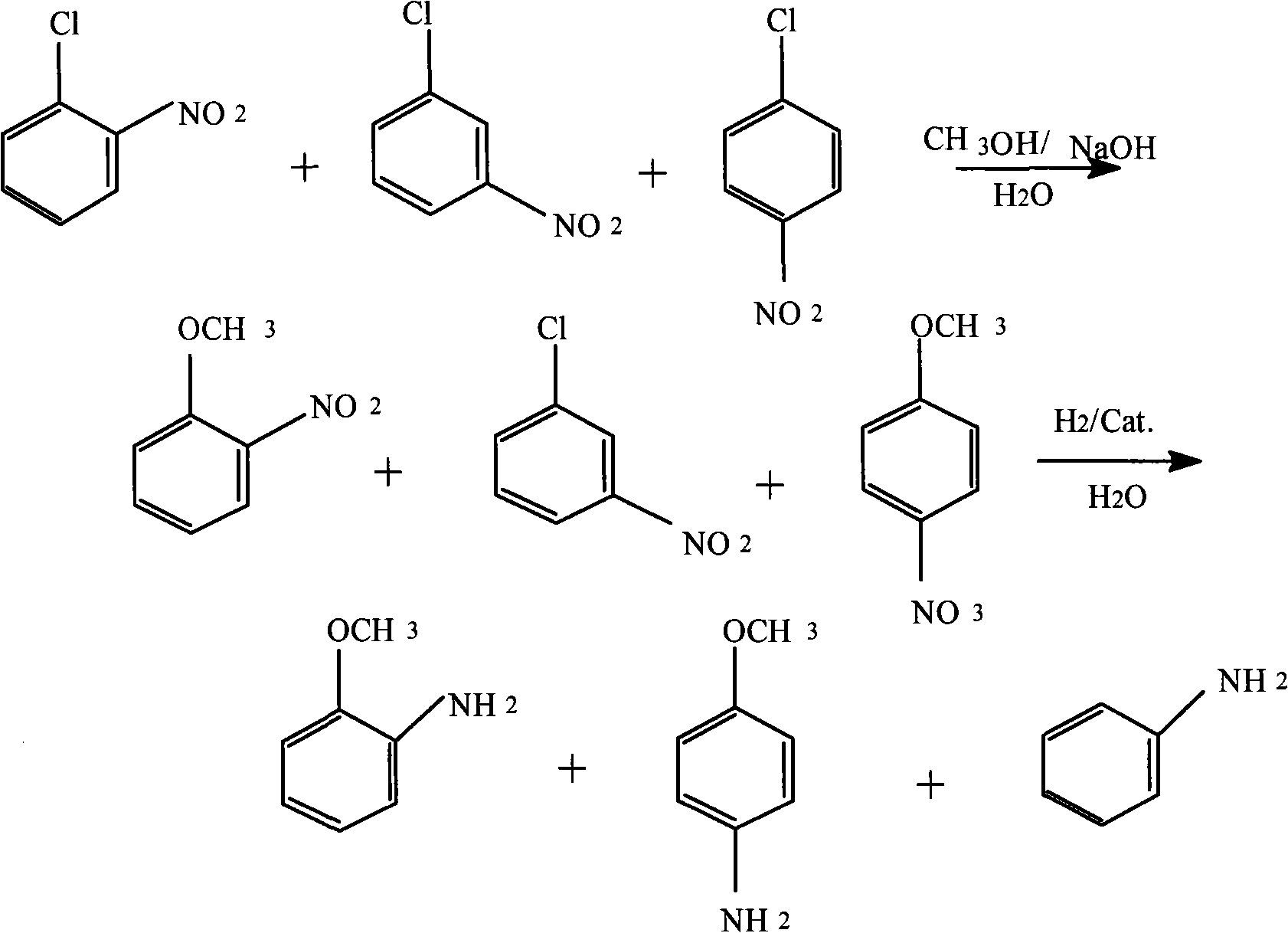
Method for producing anisidine by mixed nitrochlorobenzene reacting in aqueous solvent
InactiveCN101607919ALow ingredient requirementsReduce pollutionOrganic compound preparationAmino-hyroxy compound preparationO-nitrochlorobenzeneDistillation
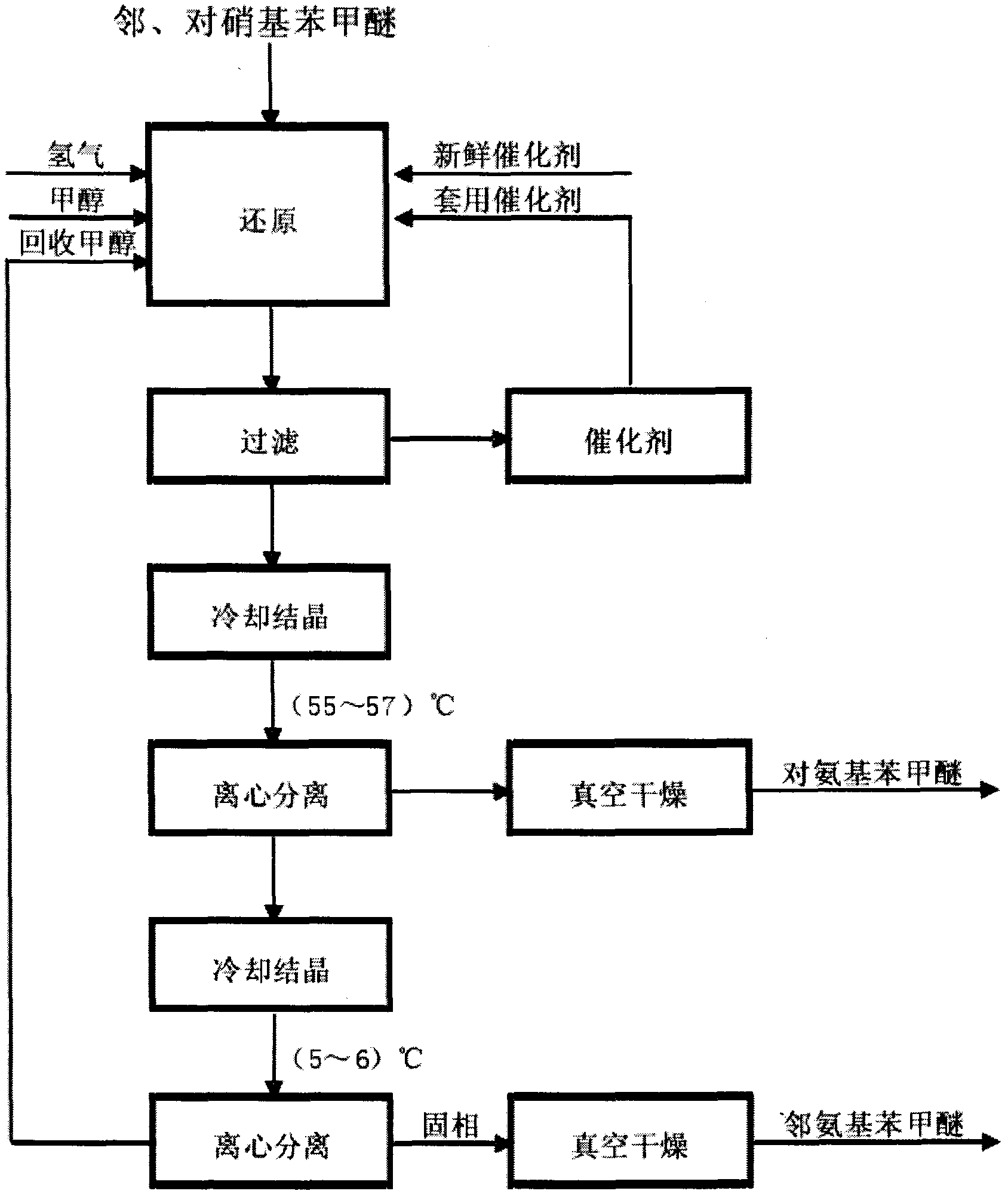
The invention relates to a method for producing anisidine by mixed nitrochlorobenzene (comprising o-nitrochlorobenzene, p-nitrochlorobenzene and m-nitrochlorobenzene) in an aqueous solvent through steps of etherification, hydrogenation, distillation separation, and the like. The method comprises the technical processes: (1) enabling the mixed nitrochlorobenzene and methanol to react, using water as a solvent and sodium hydroxide as a catalyst; (2) separating an aqueous phase; (3) catalyzing and hydrogenating etherified oil, and directly hydrogenating and reducing the etherified oil by using water as the solvent without washing to remove alkaline by water; (4) filtering the catalyst; (5) separating crude products, cooling and precipitating an organic phase, and separating and removing the water phrase; and (6) rectifying and separating an organic phase, and rectifying the organic phase obtained by separating water to obtain pure p-anisidine and pure o-anisidine with the purity over 99 percent. The method for producing anisidine by mixed nitrochlorobenzene reacting in an aqueous solvent has simple technology, low cost and energy consumption, high product purity, environmental protection and low toxicity.
Owner:扬州铭睿达化工科技有限公司 +1
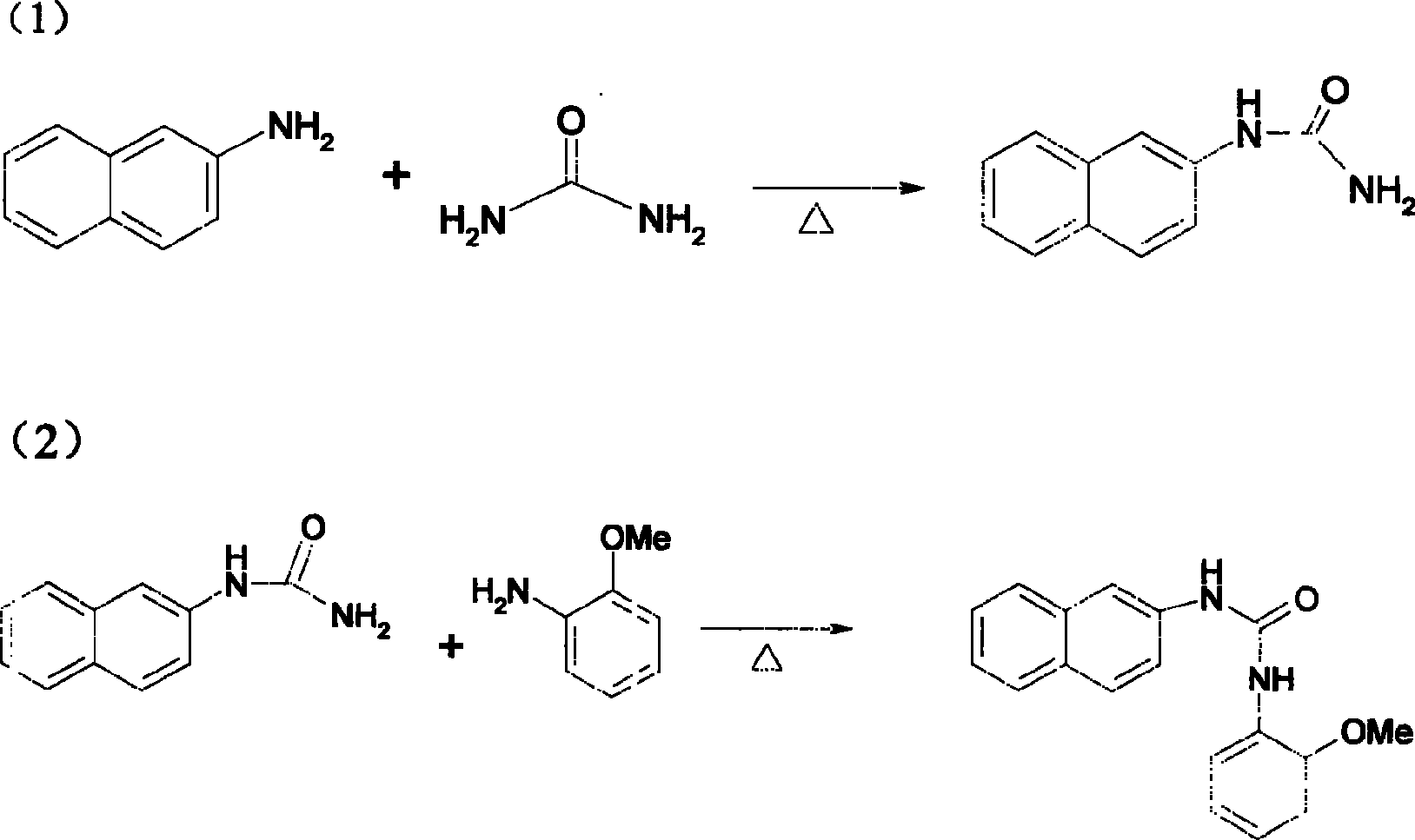
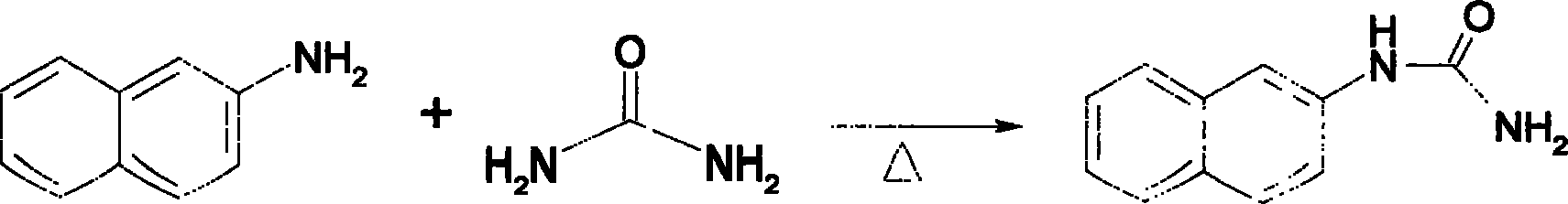
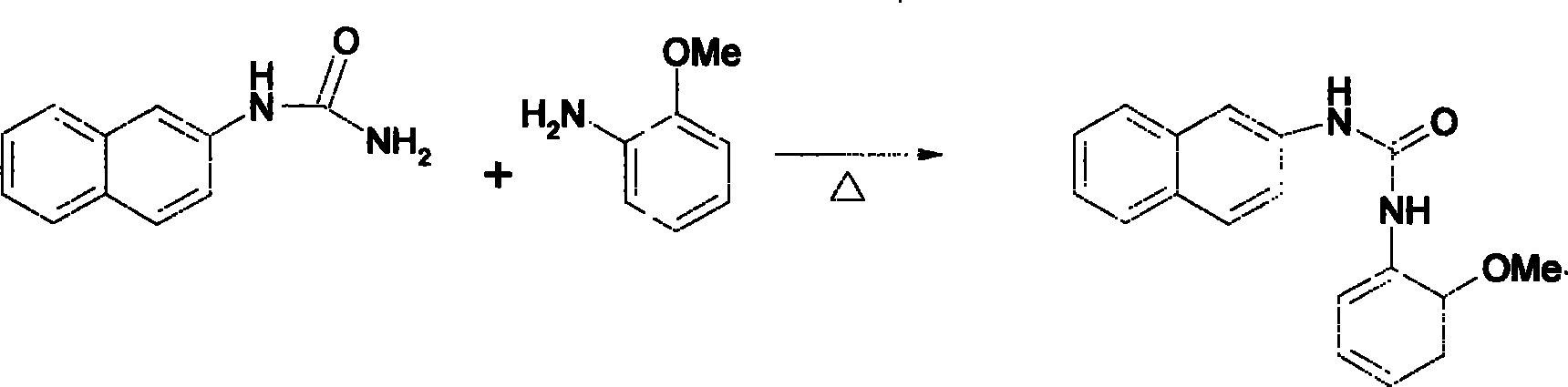
Method for synthesizing pyrazole [3,4-d] pyrimidine-4(5H)-ketone compounds
The invention relates to a method for synthesizing pyrazole [3, 4-d] pyrimidine-4(5H)-ketone heterocyclic compound, which uses o-anisidine pyrazole cyanide to be reacted with ketone, to generate target compound. The reaction formula is represented as pattern, wherein R1 and R2 are substituents, as aryl, alkyl, halide, nitro, nitroso or alkoxy, while the number and positions of the substituents are not limited, R3 and R4 are alkyl, cycloalkyl or aromatic group, the catalyst is Lewis acid, protonic acid or alkali, while the best embodiments are anhydrous zinc chloride, anhydrous aluminum chloride, copper chloride, copper chloride, alcaine, sulfuric acid, pyridine, sodium carbonate, caustic soda (or caustic potash), and sodium alcoholate (potassium alcoholate), the reaction uses generate heating method, and the purification uses recrystallization or column chromatography separation. The invention has easily accessible starting materials, simple process, mild reaction condition and wide reaction application range, which can use different substrates to synthesize various pyrazole [3, 4-d] pyrimidine-4(5H)-ketone heterocyclic compounds.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Method of preparing o-anisidine and p-anisidine through hydrogenation reduction of mixture of o-nitroanisole and p-nitroanisole
InactiveCN103073436ASimple processImprove conversion rateOrganic compound preparationChemical recyclingP-nitroanisoleO-Nitroanisole
The invention relates to a method of preparing o-anisidine and p-anisidine through hydrogenation reduction of a mixture of o-nitroanisole and p-nitroanisole. According to the method, o-nitroanisole and p-nitroanisole are used as raw materials, and o-anisidine and p-anisidine are prepared in a methanol system through hydrogenation reduction with Pt / C as a catalyst. With the method provided by the invention, the disadvantages of high energy consumption and high cost in conventional production process of o-anisidine and p-anisidine are overcome, and the technical problems of severe environmental pollution and loss of materials caused by considerable process waste water generated in the process of pretreatment in conventional production process are overcome; the method provided by the invention has the advantages of easiness, a high conversion rate, clean, green and environment-friendly process and a small amount of pollution by three wastes.
Owner:CHANGZHOU JIASEN CHEM
O-aminoanisole electrochemical synthesis method
InactiveCN101187033AMild reaction conditionsEasy to produceElectrolysis componentsElectrolytic organic productionSupporting electrolyteO-Nitroanisole
Provided is an electrochemical synthetic method of ortho-anisidine, which includes four steps that firstly the method needs to be accomplished in an two-chamber electrolysis bath which is separated by employing a cation-exchange membrane, a copper sheet is taken as a negative electrode, a ruthenium net is taken as a positive electrode, a saturated calomel electrode is taken as a reference electrode, the negative electrode and the reference electrode are installed inside a cathode chamber of the electrolysis bath, and the positive electrode is installed inside an anode chamber of the electrolysis bath. Secondly methanol is taken as solvent, sulphuric acid is taken as supporting electrolyte, ortho-nitroanisole is taken as electrolytic reaction substrate, the solvent and solution of the supporting electrolyte are injected into the cathode chamber and the anode chamber, and the electrolytic reaction substrate is injected into the cathode chamber. Thirdly the electrolyzation is performed under the condition of normal temperature and pressure and the condition that the negative electrode is added with a certain constant voltage relative to the reference electrode, the voltage value of the constant voltage is between -0.6 to -1.0V. Fourthly after the electrolyzation is finished, the electrolyte is post-processed to prepare the product of the ortho-anisidine with the production ratio between 19.1-53.4%. The method has the advantages of simple requirement, mild reaction conditions, easy preparation of the electrodes, low price, small pollution in the process of reaction and the like, which is a greening production line.
Owner:EAST CHINA NORMAL UNIV
O-methoxy-beta-sulfuric ester ethyl sulfonyl aniline and preparation method thereof
InactiveCN101602699AHigh effective contentImprove product qualityOrganic chemistryOrganic compound preparationAcetic anhydrideChlorosulfuric acid
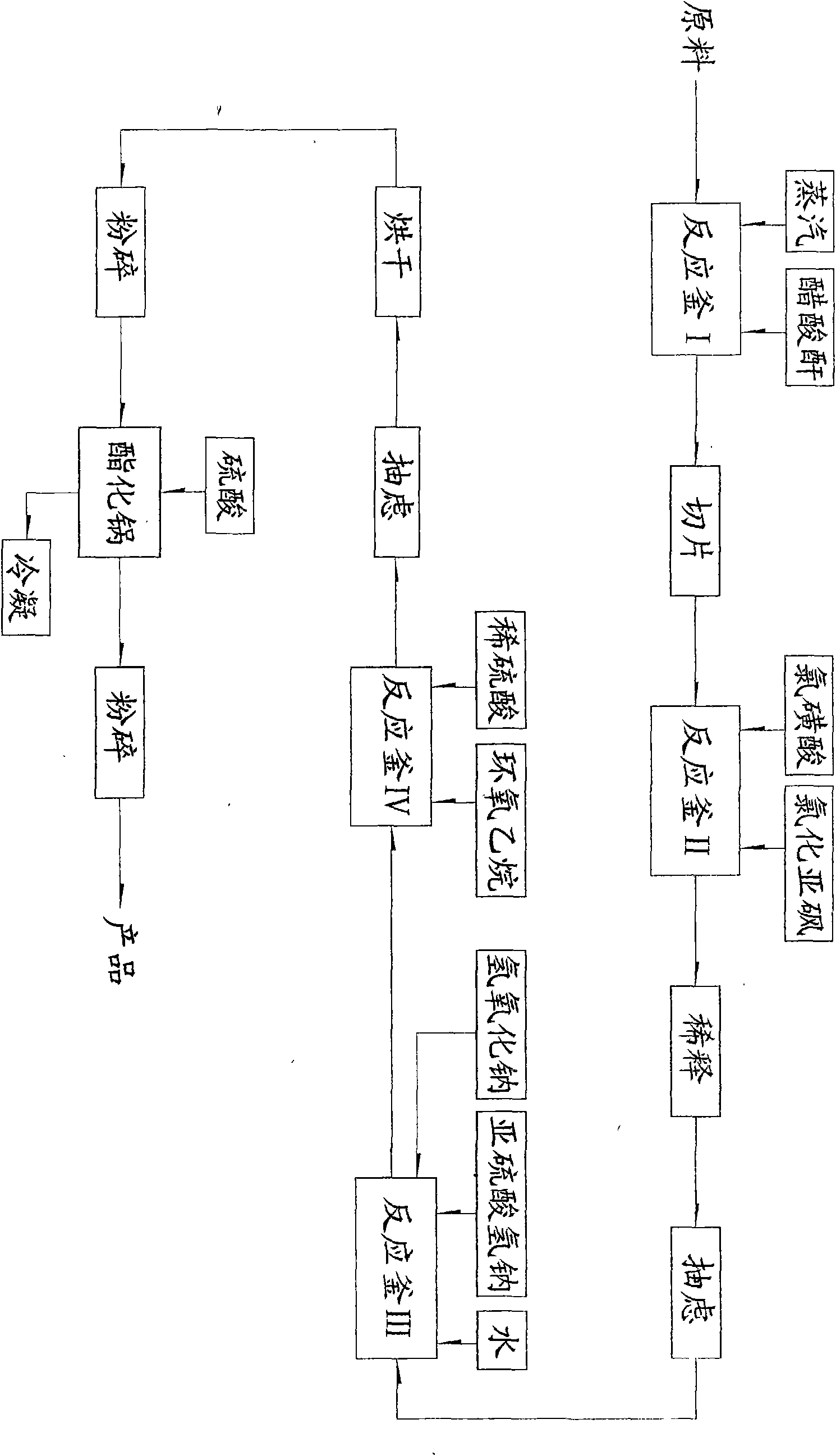
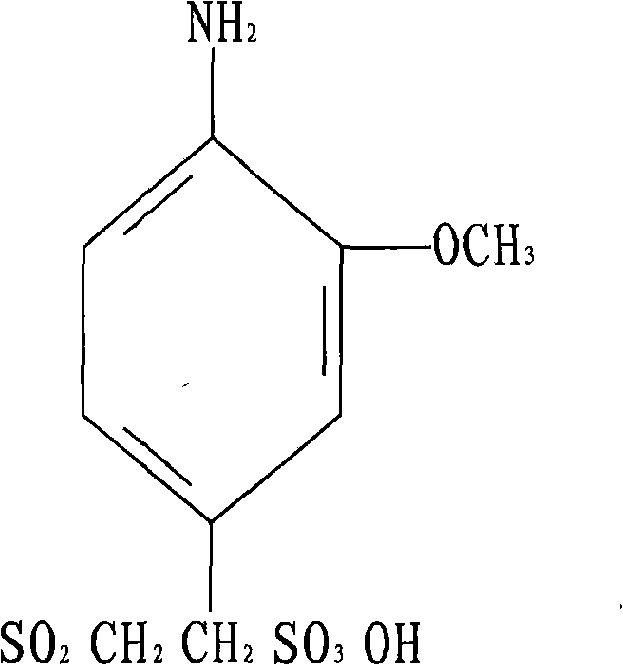
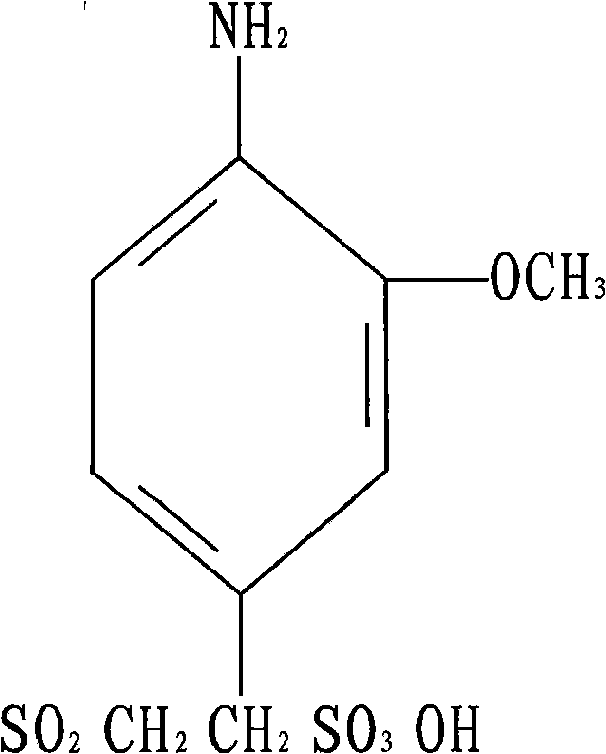
The invention discloses o-methoxy-beta-sulfuric ester ethyl sulfonyl aniline and a preparation method thereof. The o-methoxy-beta-sulfuric ester ethyl sulfonyl aniline is used as a raw material for synthesizing dyes such as reactive blue 250, and the like, and is prepared according to the following steps: o-aminoanisole is acetylized by using acetic anhydride solution; the obtained acetylized compound is sulfonated by using chlorosulfuric acid and thionyl chloride; the obtained sulfonate is reduced by using sodium bisulfite and sodium hydroxide; the reducing substances are condensed by using ethylene oxide; and the obtained condensation compound is esterified by using sulphuric acid to obtain the product. The reactions are realized by mixing, dissolution and stirring in a reaction container and adopting the methods of vacuum heating, slicing, diluting, clarification, washing, suction filtering, drying and crushing. The invention solves the problems that the prior product depends on import and has high use cost and complex process, and has the advantages of simple manufacture method, easy operation and high effective content up to more than 97 percent.
Owner:韩朝忠 +1
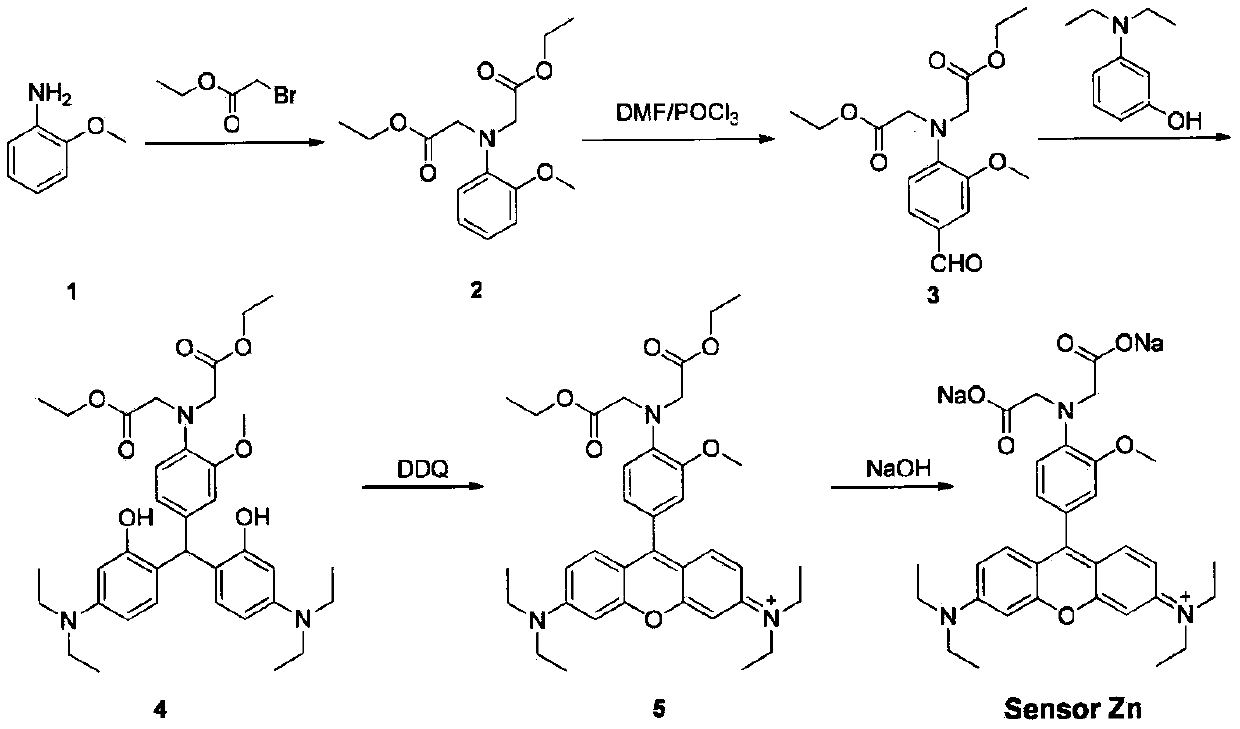
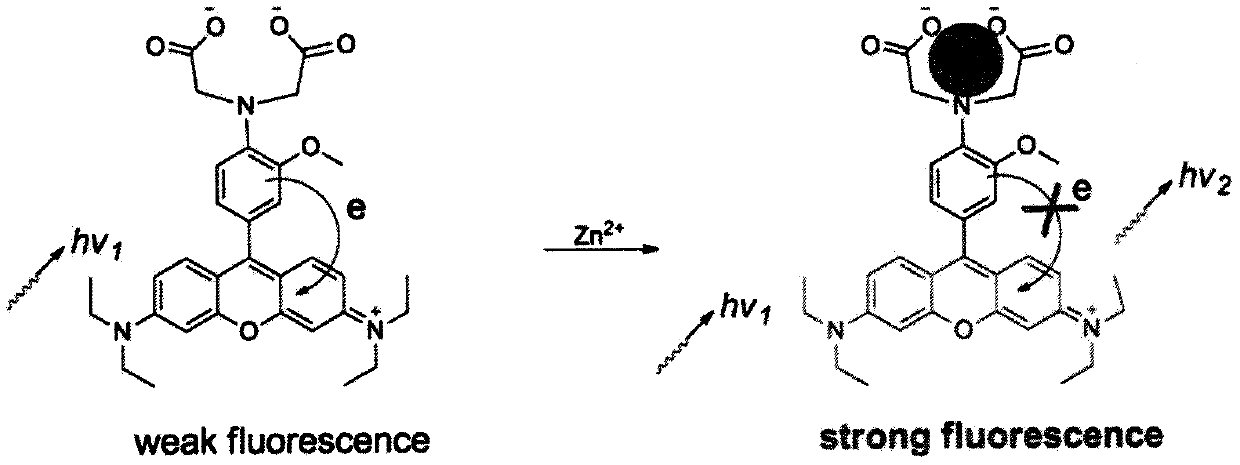
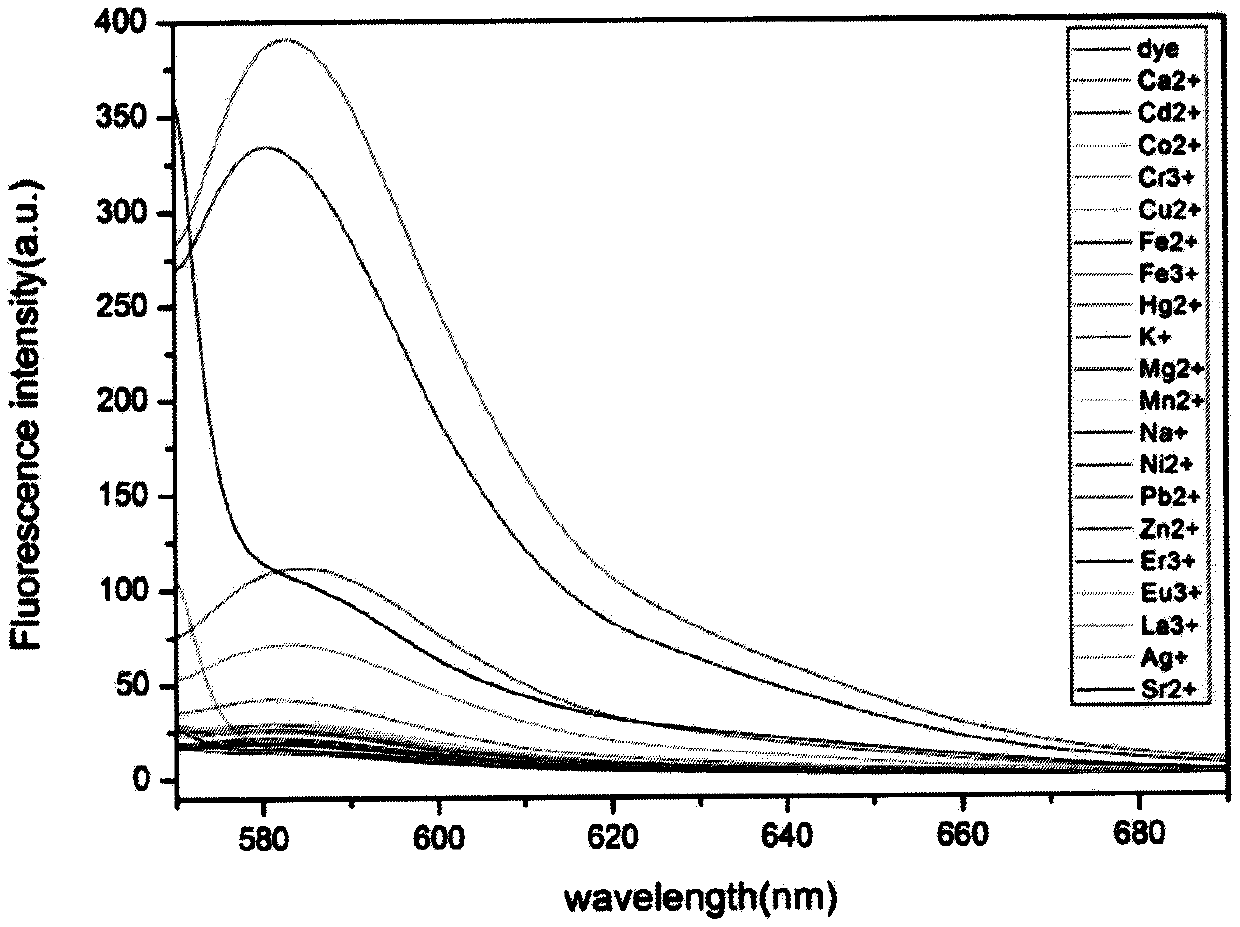
Fluorescent compound for detecting metal ion content in water, and applications thereof
InactiveCN110128394AContent calculationRealize non-invasive in situ detectionOrganic chemistryFluorescence/phosphorescenceIon contentChemical compound
The invention discloses a fluorescent probe for detecting the metal ion content in water, wherein a rhodamine fluorophore is introduced into the molecule of N-o-anisidine acetate used as a metal ion complex to generate the fluorescent indicator for metal ions, particularly zinc ions. According to the present invention, the compound can further be used for in situ imaging of cells, can be used forthe continuous detection of metal ion concentrations in various environments, and especially can be used for the continuous determination of the zinc ion concentration in water.
Owner:TIANJIN AGRICULTURE COLLEGE
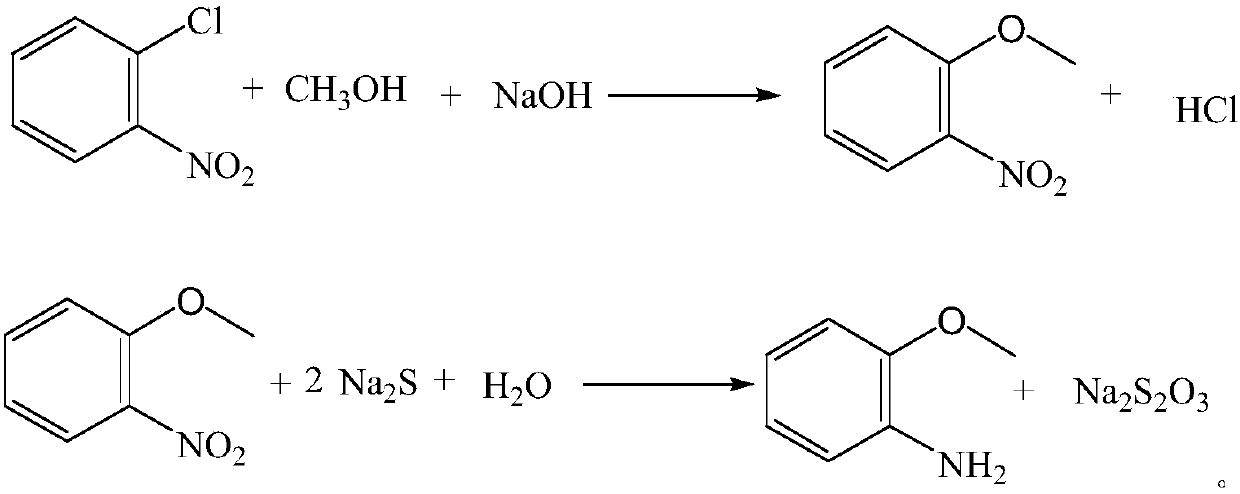
Production process for o-aminoanisole and preparation method thereof
InactiveCN107673981AEasy to getSimple production processOrganic compound preparationChemical recyclingO-NitroanisoleWastewater
The invention discloses a production process for o-aminoanisole. The production process for the o-aminoanisole comprises the following steps: carrying out etherification by using methanol and sodium hydroxide as methoxyl reagents to obtain o-nitroanisole; and reducing the o-nitroanisole by sodium sulphide under the effect of a catalyst to synthesize the o-aminoanisole. According to specific reaction, main reaction is as shown in specification. In the production process for the o-aminoanisole, production raw materials are easily obtained; the production process is simple and easy; the problemsof difficulty in recycling of the catalyst and serious fire in hydrogenation reduction are solved, a certain amount of waste water is generated by reduction through sodium sulfide, and the productionprocess for the o-aminoanisole has the advantages of good product quality and high safety factors.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
Method for determining six aromatic amine compounds in cigarette mainstream smoke by using online SPE/LC-MS/MS
ActiveCN106290687AEasy to handleAutomate the processComponent separationOnline speDimethylaniline N-oxide
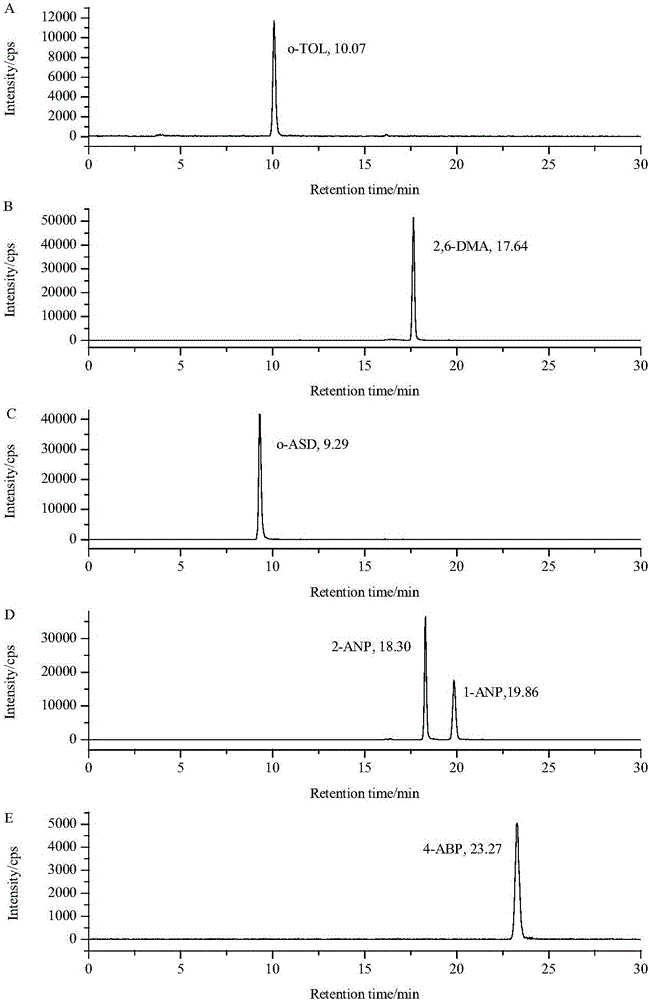
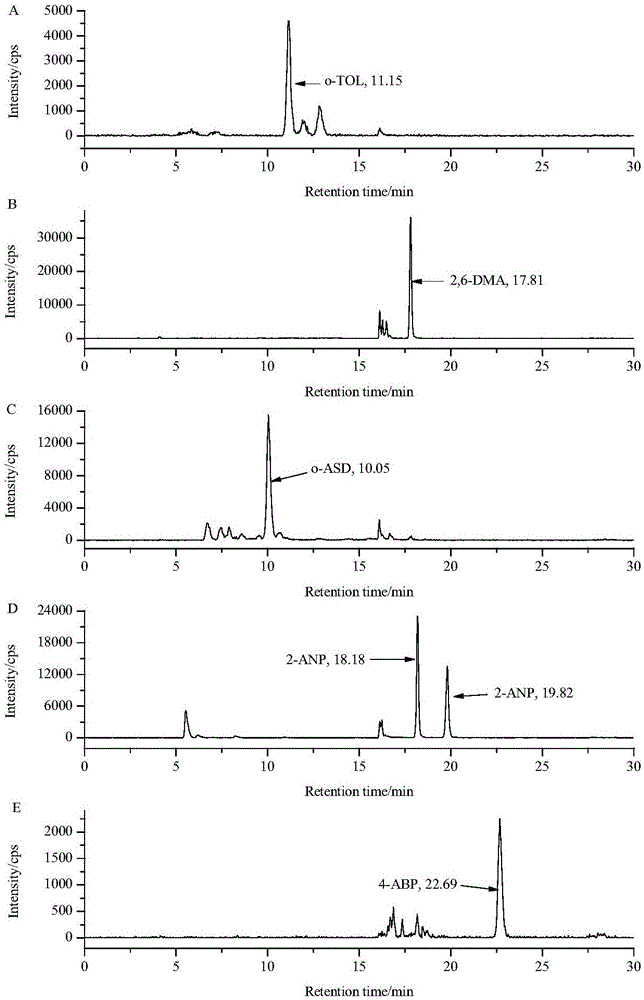
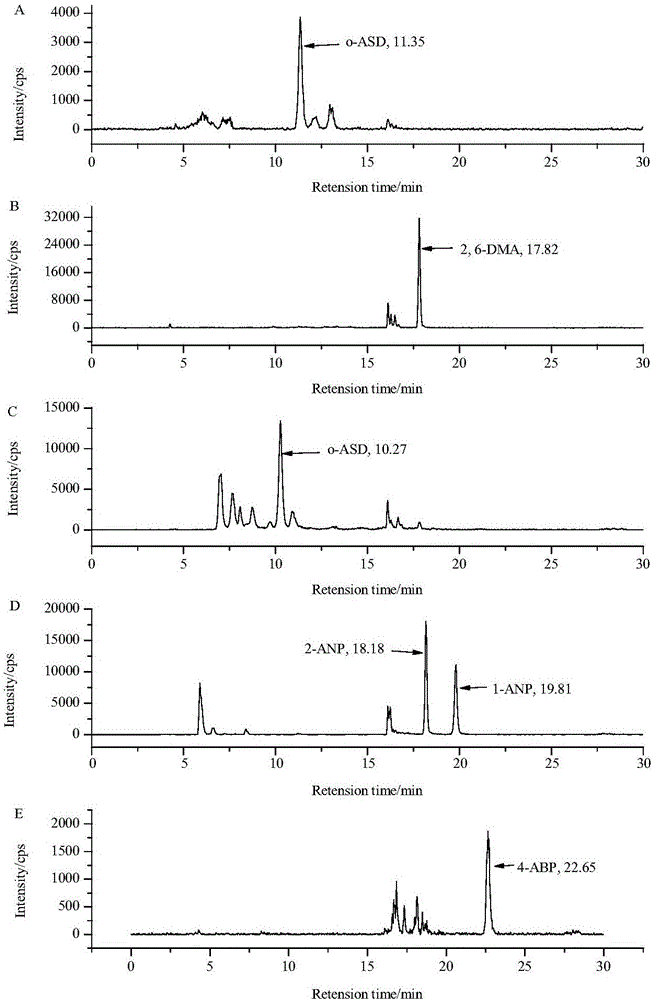
The invention discloses a method for determining six aromatic amine compounds in cigarette mainstream smoke by using online SPE / LC-MS / MS. It uses the Cambridge filter and an absorption bottle containing 2% formic acid solution to capture the aromatic amine compounds in the main cigarette smoke particle and gas phase matters, respectively, ultrasonically extracting the filter by 2% formic acid solution, adding interior labels into the two solutions and determining the six aromatic amine compounds by online SPE / LC-MS / MS, including o-toluidine, 2,6-dimethylaniline, o-anisidine, 1-aminonaphthalene, 2-aminonaphthalene and 4-aminobiphenyl. The method has the advantages of simplifying the sample pretreatment process and extracting with the formic acid solution to reduce the pollution. The two-dimensional in-line solid-phase extraction composed of the cationic column and the reversed-phase C18 column is used to purify the sample to be tested and the purification effect is good; each target is quantitatively analyzed using their specific internal standard, effectively reducing the matrix effect.
Owner:中国烟草总公司山东省公司
Method for preparing m-chloroaniline by using meta-position oil
ActiveCN106883129ALow costHigh outputOrganic compound preparationAmino compound preparationSodium hydrosulfideToxic industrial waste
The invention relates to a method for preparing m-chloroaniline by using meta-position oil and belongs to the technical field of preparation of m-chloroaniline. The method specifically comprises the steps of carrying out an ingredient reaction, carrying out water washing, carrying out drying, carrying out third-stage vacuum distillation, carrying out crystallization, carrying out reduction and carrying out re-distillation. According to the method, m-chloroaniline is prepared from waste, i.e., meta-position oil of production of p-nitrophenol and o-nitrophenol and industrial waste liquid, i.e., sodium hydrosulfide, which serve as raw materials, by using methoxylation, crystallization and reduction, and byproducts, i.e., o-aminoanisole and p-aminoanisole are produced, so that low cost and high yield are achieved, and the method has an obvious cost advantage and higher economic benefit compared with the existing production technologies.
Owner:ANHUI HAIHUA CHEM
Preparation method for red base B
ActiveCN105061232ALow priceAtom utilization is highOrganic compound preparationAmino-hyroxy compound preparationFormylation reactionFiltration
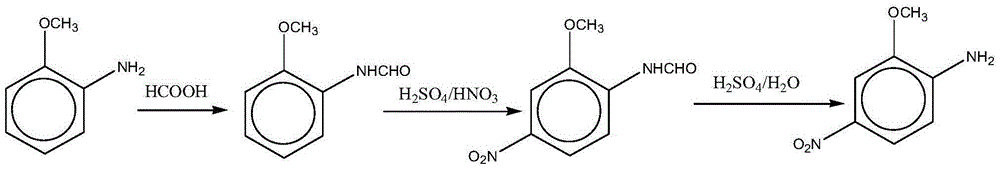
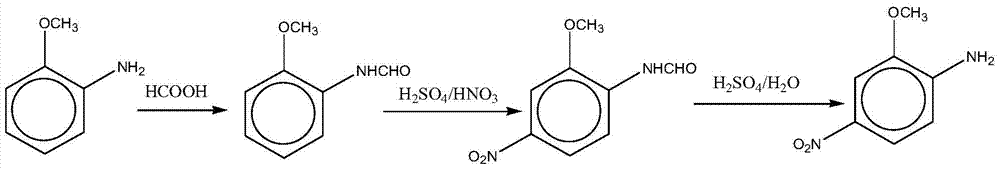
The invention relates to a preparation method for a red base B. A formylation reaction is performed on o-aminoanisole and formic acid at 80-102 DEG C on the condition that acylation reaction solvent exists, and filtration is performed to obtain o-formamide anisole after the formylation reaction is completed; the o-formamide anisole is added into a sulfuric acid solution, nitric acid is added into a reaction system to perform a nitration reaction when the temperature of the reaction system is controlled at 0-70 DEG C, and filtration is performed to obtain methoxyl-4-nitroformanilide after the nitration reaction is completed; a hydrolysis reaction is performed on the methoxyl-4-nitroformanilide on the condition that the sulfuric acid and water exist at 80-120 DEG C, after the hydrolysis reaction is completed, separation is performed, the pH value is regulated to 6-7 with sodium carbonate, and then filtration is performed to obtain the red base B. According to the preparation method, the produce yield is high, the cost is low, the quality is good, by-products are less, waste water generated in the production process of the red base B is greatly reduced, the clean production requirement is met, operation is easy and convenient, and industrialization is facilitated.
Owner:XIANGSHUI HENRYDA TECH CHEM
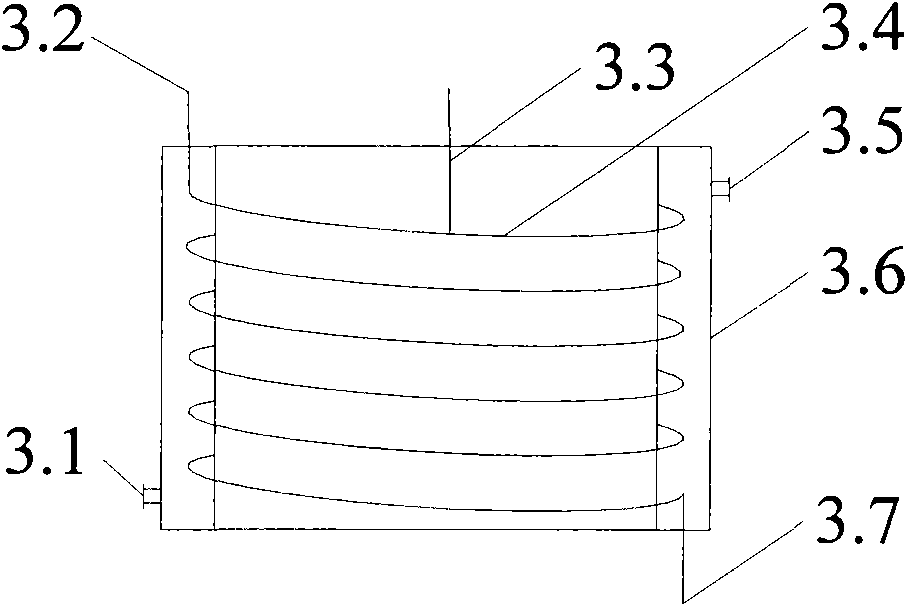
Technique and installation for preparing guaiacol by continuously hydrolyzing diazonium salt of o-amino pheylmethyl ether
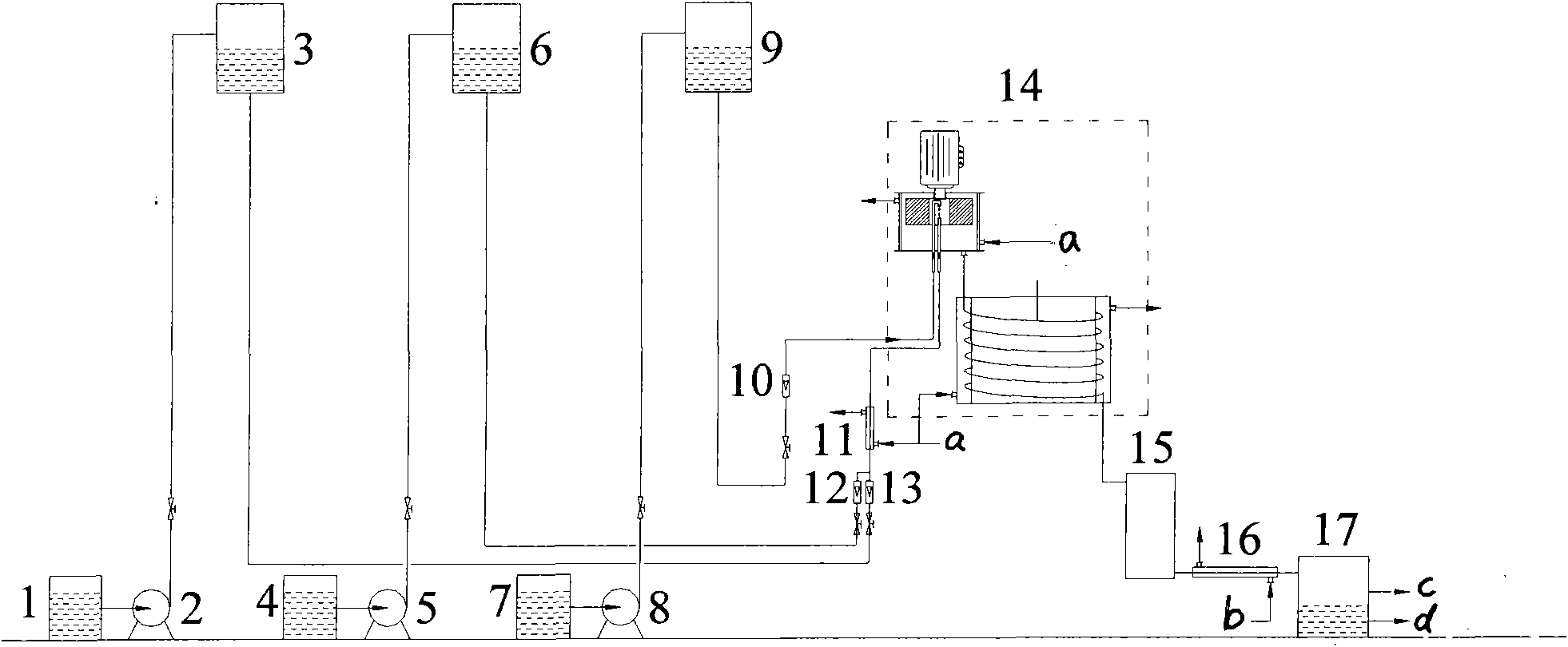
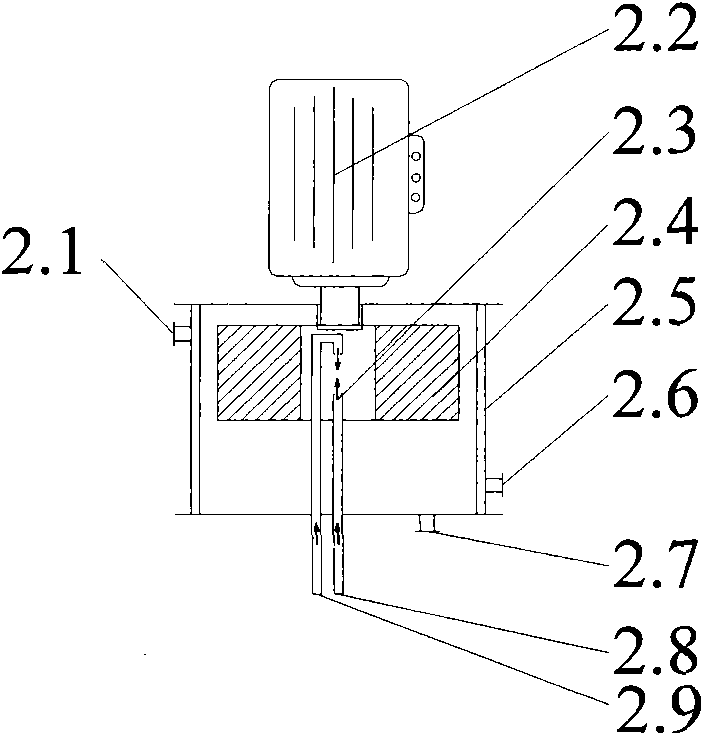
ActiveCN101774895AQuick mixDisperse fastOrganic chemistryOrganic compound preparationHigh energyHydrolysate
The invention belongs to the technical field of guaiacol preparation, in particular to a technique and an installation for preparing guaiacol by continuously hydrolyzing diazonium salt of o-amino pheylmethyl ether, which solves the problems of the prior guaiacol production method, including low efficiency, high energy consumption, severe pollution and difficult continuous production. The installation comprises a hydrolysis reactor which consists of a rotating packed bed and a coil, the coil is axially vertically arranged under the rotating packed bed, a liquid outlet of the rotating packed bed is connected with a coil inlet, and a coil outlet is sequentially connected with a stirrer, a condenser and an output liquid storage tank. The method includes the following steps that: hydrolysate and extractant are mixed and preheated, and are then sent into the hydrolysis reactor along with the diazonium salt solution of o-amino pheylmethyl ether at the same time and heated to the hydrolysis temperature, so that the guaiacol is produced. The invention has the following advantages that: the production of by-products can be reduced, the yield is increased to more than 90 percent, the emission of the three wastes is reduced, the production cost is reduced, moreover, the equipment size is small, the startup / shutdown time is short, and mounting, operation and maintenance are convenient.
Owner:ZHONGBEI UNIV
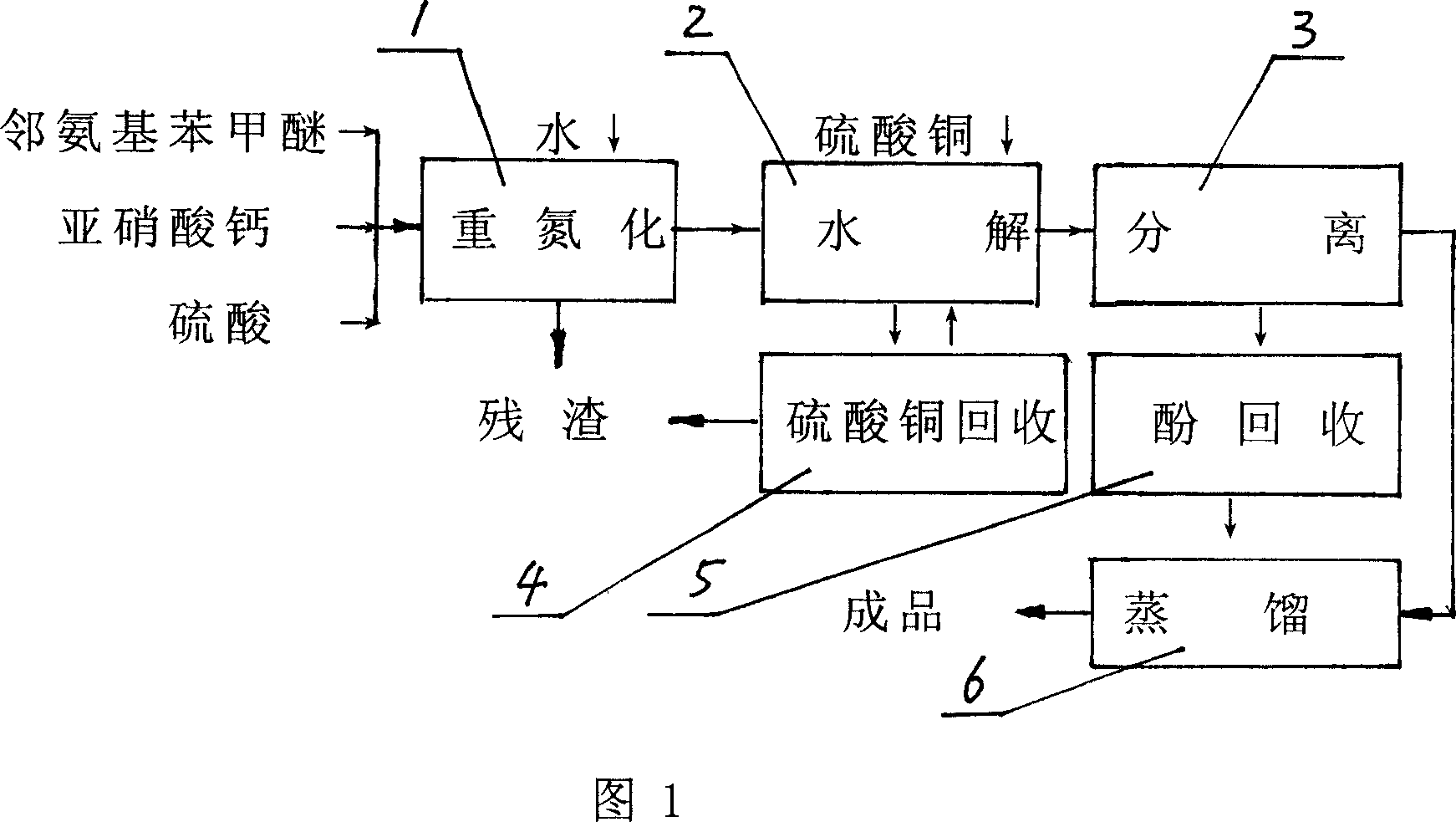
Method of preparing methyl catechol using calcium nitrite as raw material
InactiveCN1948252AHigh yieldImprove hydrolysis conditionsOrganic chemistryOrganic compound preparationDistillationGuaiacol
The present invention relates to a method for preparing guaiacol by using calcium nitrite as raw material. Said method is characterized by that it includes the following steps: in a diazotization still adding o-aminoanisole into dilute sulfuric acid to make neutralization to obtain methyl ether sulfate, then drop-adding calcium nitrite solution to make diazotization reaction, separating out solid residue, feeding the diazotization solution into hydrolysis procedure to make hydrolysis reaction so as to obtain crude guaiacol, then feeding the obtained crude guaiacol into separation procedure; making the waste liquor be fed into copper sulfate recovery procedure and making the phenol water containing crude guaiacol be fed into phenol recovery procedure, making the phenol water be continuously adsorbed in adsorption tower with macroporous adsorbing resin, using sodium hydroxide solution to make elution, using sulfuric acid to acidify eluent to separate out crude guaiacol; mixing above-mentioned two crude guaiacols in a distillation still, making reduced pressure distillation to remove water content and impurity from crude guaiacol so as to obtain the invented guaiacol finished product.
Owner:刘仁杰
Method for synthesizing o-aminoanisole by hydrogenation method
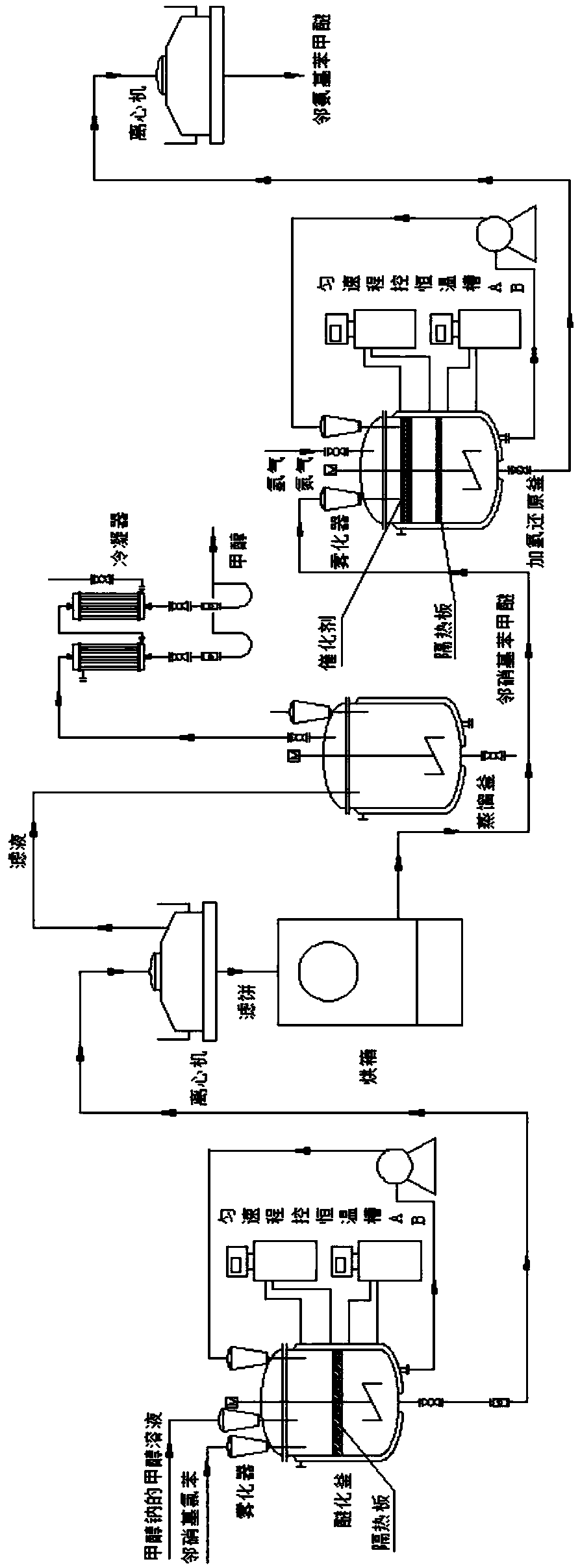
InactiveCN109053472AIncrease contact areaHigh purityOrganic compound preparationAmino-hyroxy compound preparationO-nitrochlorobenzeneSodium methoxide
The invention discloses a method for synthesizing o-aminoanisole by a hydrogenation method. The synthesis method specifically comprises the following steps: adding metallic sodium to excess methanol to prepare a methanol solution of sodium methoxide, and then spraying o-nitrochlorobenzene and the methanol solution of sodium methoxide into an etherification kettle; firstly, performing centrifugal separation, then transferring to a distillation kettle for distillation, and then crystallizing and filtering; firstly, introducing the hydrogen gas to exhaust the gas, atomizing o-nitroanisole, usinga catalyst for catalyzing the reaction, introducing nitrogen gas to the kettle to exhaust the gas after the reaction is completed, then cooling and crystallizing, centrifuging at low temperature for separation and filtering, and repeatedly operating for 2 to 3 times to obtain o-aminoanisole. The method for synthesizing o-aminoanisole by the hydrogenation method uses nitrogen-doped porous carbon asa carrier for the catalyst, and the catalyst is made into a lattice. Contact area of the reactant is large, and the reaction proceeds rapidly. A methoxy reagent is directly prepared from the metallicsodium and methanol, and the methoxy reagent is dissolved with methanol to prepare a solution for spraying, so as to accelerate the reaction and promote the reaction to proceed forward.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
Synthesis method of o-amino pheylmethyl ether
InactiveCN104086448AEasy to useEasy to prepareOrganic compound preparationAmino-hyroxy compound preparationChemical industryO-nitrochlorobenzene
The invention discloses a preparation method of o-amino pheylmethyl ether, and relates to the technical field of chemical industry. The method comprises the following steps: adding o-chloronitrobenzene, methanol and a 40-percent sodium hydroxide solution into a high-pressure reaction kettle in sequence, raising the temperature in the kettle to 40 DEG C, and stirring; raising the temperature to 85 DEG C, controlling the pressure at 0.28-0.32MPa, reacting for 8 hours, distilling, removing an internal methanol solution, adding hot water of 70 DEG C for washing, standing for delaminating, and performing liquid separation to obtain o-nitroanisole for later use; putting o-nitroanisole into the high-pressure reaction kettle, adding a sodium sulfide aqueous solution, controlling the pressure at 0.05MPa, controlling the temperature at 118-120 DEG C, pressurizing, refluxing, cooling to 50-60 DEG C, preserving heat for 5 hours, performing liquid separation, removing internal waste water, distilling, crystalizing, drying to obtain finished o-amino pheylmethyl ether, packaging and warehousing. The preparation method has the beneficial effects of convenience and easiness in preparation, environmental friendliness, pollution freeness, ready availability of raw materials, small equipment investment, high purity and convenience in operation. The prepared o-amino pheylmethyl ether has a good use effect, and is safe and reliable.
Owner:安徽佑骏商品混凝土有限公司
Composition containing attractant of noxious arthropod comprising plant-derived component and analogue of same
InactiveUS20160227776A1Superior noxious arthropod attracting effectEfficient captureBiocideDrug compositionsBenzaldehydeEugenol
Owner:KYOYU AGRI
Production method of oamino pheylmethyl ether
ActiveCN102276483BReduce consumptionSimple processOrganic compound preparationAmino-hyroxy compound preparationO-nitrochlorobenzeneSodium methoxide
The invention provides a production method of oamino pheylmethyl ether, and the method comprises the following steps of: reacting ortho nitrochlorobenzene serving as a raw material with sodium methoxide for carrying out a methoxylation reaction to obtain orthonitroanisole; secondly, carrying out hydrogenation reduction on the orthonitroanisole by using methanol as a solvent in the presence of a catalyst to prepare the oamino pheylmethyl ether; and finally, carrying out dealcoholization, dehydrogenation and refining on reactants to obtain the oamino pheylmethyl ether as a finished product. The production process comprises the following steps of: preparing sodium methoxide, etherifying the ortho nitrochlorobenzene, distilling the methanol and nitroether for separation, hydrogenating the orthonitroanisole, distilling a hydrogenating solution for separating the oamino pheylmethyl ether and treating wastewater. The production method has the characteristics of simple process, short procedure, continuity in reaction, high production efficiency, good product quality, less energy consumption, concentrated purification of reaction wastewater and no emission, and is suitable for producing the oamino pheylmethyl ether by using the ortho nitrochlorobenzene as the raw material.
Owner:LIAONING SHIXING PHARMA & CHEM
Technology for preparing hydroquinone through diazotation hydrolysis method
InactiveCN104086372AEasy to useEasy to prepareOrganic chemistryOrganic compound preparationChemical industryThiourea
A technology for preparing hydroquinone through a diazotation hydrolysis method relates to the technical field of the chemical industry. The technology comprises the following steps: reacting a diazo liquid obtained after a reaction of sulfuric acid, p-aminophenol, sodium nitrite, urea and thiourea with sulfuric acid with the concentration of 30%, extracting, and processing to obtain hydroquinone. The technology has the advantages of convenient and simple preparation, environmental protection, no pollution, easily available materials, less equipment investment, high purity and convenient operation, and the prepared hydroquinone has the advantages of good use effect, safety and reliability.
Owner:安徽佑骏商品混凝土有限公司
A kind of preparation method of red base b
ActiveCN105061232BLow priceAtom utilization is highOrganic compound preparationAmino-hyroxy compound preparationFormylation reactionNitration
The invention relates to a preparation method of red base B. In the presence of an acylation reaction solvent, o-aminoanisole and formic acid are subjected to formylation reaction at 80-102°C, and o-formamide is obtained by filtration after the reaction Anisole: o-formamide anisole is added to the sulfuric acid solution, and when the temperature of the reaction system is controlled at 0-70°C, nitric acid is added to the reaction system for nitration reaction, and after the reaction is completed, it is obtained by filtration 2-methoxy-4-nitroformanilide; 2-methoxy-4-nitroformanilide is hydrolyzed at 80~120°C in the presence of sulfuric acid and water. After the reaction, Separation, adjusting the pH to 6-7 with soda ash, and then filtering to obtain the red base B. The product of the invention has high yield, low cost, good quality and few by-products, greatly reduces the waste water produced in the production process of the red base B, meets the requirements of clean production, is easy to operate, and is convenient for industrialization.
Owner:XIANGSHUI HENRYDA TECH CHEM
Method for preparing o-anisidine by catalytic hydrogenation
InactiveCN102391134AReduce energy consumptionHigh yieldOrganic compound preparationAmino-hyroxy compound preparationO-NitroanisolePhysical chemistry
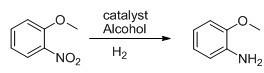
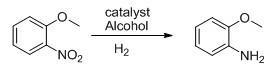
The invention discloses a method for preparing o-anisidine by catalytic hydrogenation. The method comprises the following steps of: mixing o-nitroanisole, methanol and a catalyst Raney-Ni, adding the mixture into a pressure kettle, performing replacement by hydrogen, introducing the hydrogen to maintain the pressure of between 0.5 and 2MPa, heating to the temperature of between 40 and 80 DEG C, introducing the hydrogen continuously for 3 to 10 hours, recovering the methanol, separating liquid and rectifying to obtain the o-anisidine. The method has the advantages of environment friendliness, low energy consumption, high yield and high quality of products.
Owner:JIANGSU KANGHENG CHEM
Continuous synthesis method of 2-acetamido-5-nitroanisole
ActiveCN113582867ALow costSimple and fast operationOrganic compound preparationChemical/physical/physico-chemical microreactorsOrganic synthesisNitration
The invention mainly relates to the field of organic synthesis, and discloses a continuous synthesis process of 2-acetamido-5-nitroanisole. According to the process, a micro-channel continuous flow reactor is used as main reaction equipment, o-aminoanisole is used as a starting raw material, and continuous synthesis of 2-acetamido-5-nitroanisole is realized through pre-acylation, amidation and nitration. According to the process, a composite amidation reagent is adopted, so that the cost of raw materials is reduced, and the amidation reagent and a reaction solvent are unified; a continuous production process and equipment are introduced, so that continuous production is realized, the automation degree is improved, and the production safety risk is greatly reduced; and the reaction time, the generation of byproducts and the subsequent treatment difficulty are reduced, the nitration selectivity is improved, the win-win situation of economic benefits and environmental benefits is finally realized, and the development concept of green chemistry is met.
Owner:SHANDONG NORMAL UNIV EXPERIMENTAL PLANT CO LTD +1
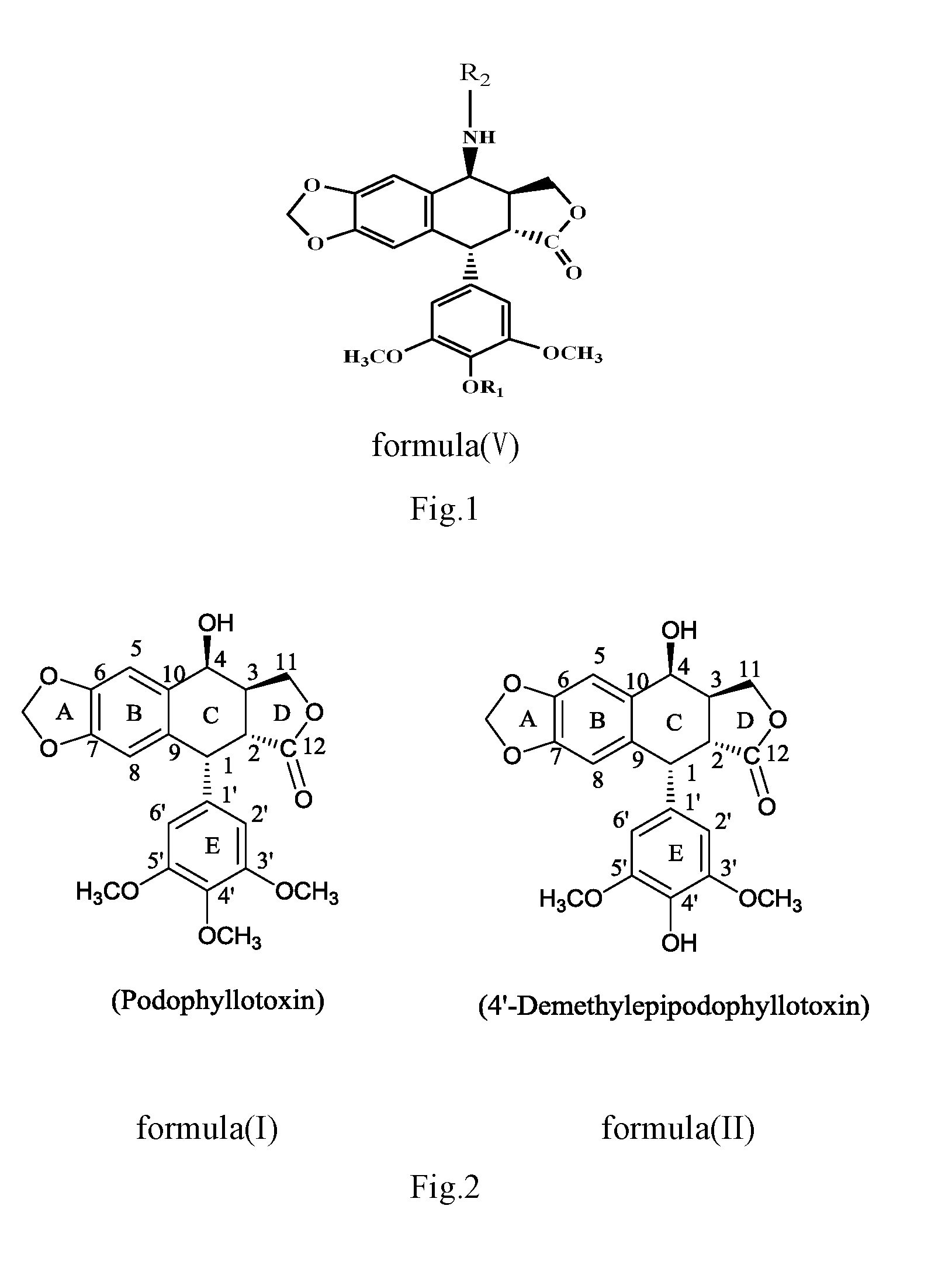
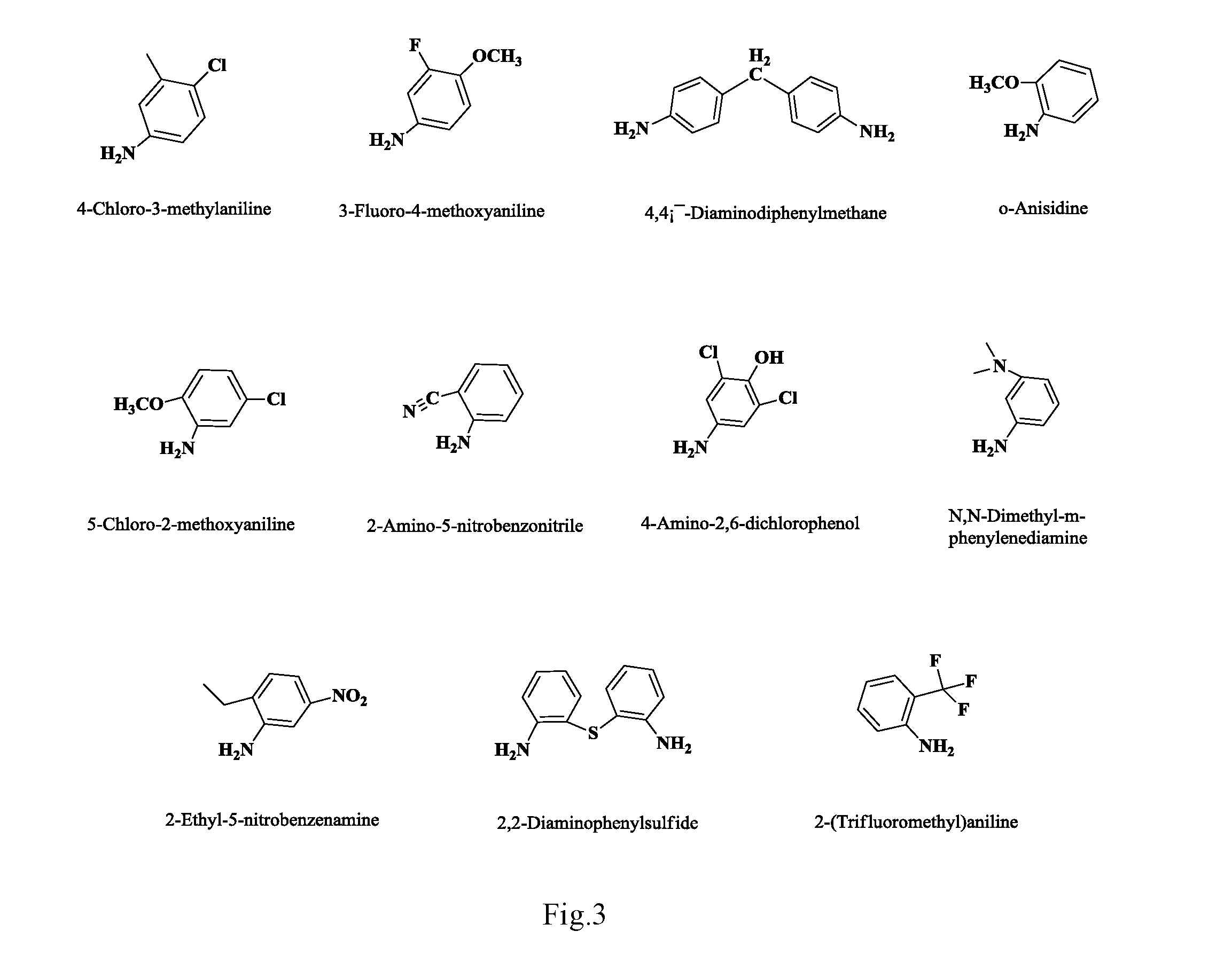
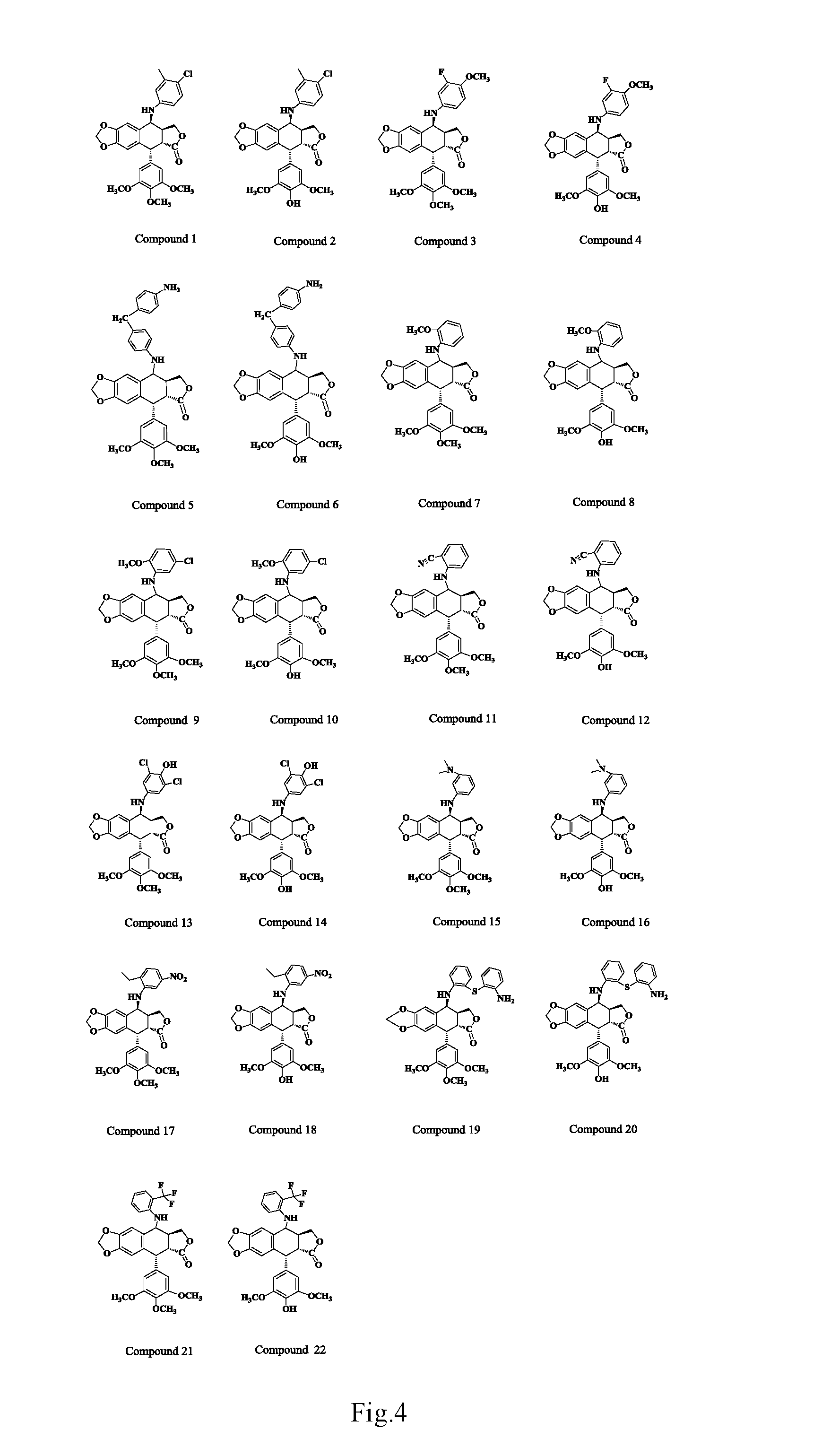
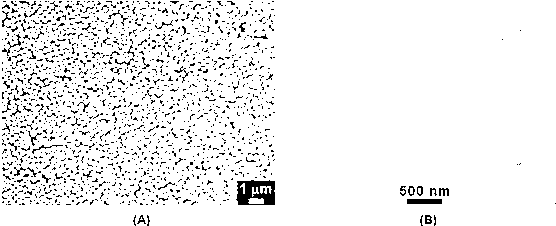
Anilino podophyllin derivative having antitumor activity, method for preparation thereof, and use thereof
InactiveUS20160289242A1Good antitumor activityImprove anti-tumor effectOrganic active ingredientsOrganic chemistryMethylanilinePodophyllin derivative
The present invention discloses an anilino-substituted podophyllin derivative having antitumor activity, method for preparation thereof, and use thereof. By means of anilino reactions, the present invention introduces 4-chloro-3-methylaniline, 3-fluoro-4-methoxyaniline, 4,4′-diaminodiphenylmethane, o-anisidine, 4-chloro-2-aminoanisole, anthranilonitrile, 2,6-dichloro-4-aminophenol, N,N-dimethylamino meta-aniline, 2-ethyl-5-nitroaniline, 2 2′-diaminodiphenylsulfide, or 2-aminobenzotrifluoride into position 4 of the active C-ring of podophyllotoxin or 4′-demethylepipodophyllotoxin to obtain the aniline-substituted podophyllotoxin derivative shown in formula (V); by means of multi-pathway and multi-target effects on tumor cells, said derivative has significantly increased antitumor activity, and can be prepared as an antineoplastic drug and applied in clinical antitumor therapy.
Owner:HUBEI UNIV OF TECH
Template-free preparation method of polymer hollow colloidal sphere
InactiveCN103100359AReduce dosageStructure does not affectChemical recyclingMicroballoon preparationPolymer scienceNanostructure
The invention relates to a template-free preparation method of a polymer hollow colloidal sphere with a nano structure. The template-free preparation method comprises the specific steps of: preparing a cupric acetate water solution as an initiator; adding the cupric acetate water solution into a stainless steel reaction kettle with a tetrafluoroethylene lining; or adding the prepared cupric acetate water solution into the stainless steel reaction kettle with the tetrafluoroethylene lining; then adding hexadecyl trimethyl ammonium bromide into the reaction kettle to prepare an alkaline copper bromide as the initiator; preparing a macromolecular monomer solution under an agitating condition; then adding the macromolecular monomer solution into the reaction kettle filled with the initiator in the step (1) to be mixed; sealing and heating to 150-180 DEG C and reacting for 4-12 hours, and cooling to obtain a colloidal solution; centrifuging and separating the colloidal solution and washing by utilizing a polarity organic solvent which is soluble to water; and dispersing the polarity organic solvent into the water again to obtain the polymer hollow colloidal sphere. The polymer hollow colloidal sphere comprises poly(o-anisidine), poly(o-ethoxyaniline), poly(3, 5-dimethoxyaniline) and polyaniline. The preparation method of the polymer hollow colloidal sphere provided by the invention is suitable for large-scale industrial production and has a very wide market prospect.
Owner:NANJING UNIV OF TECH
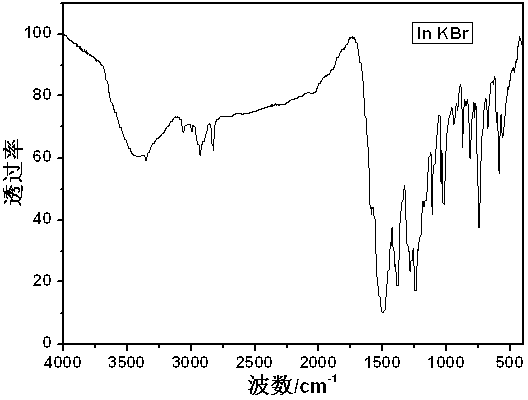
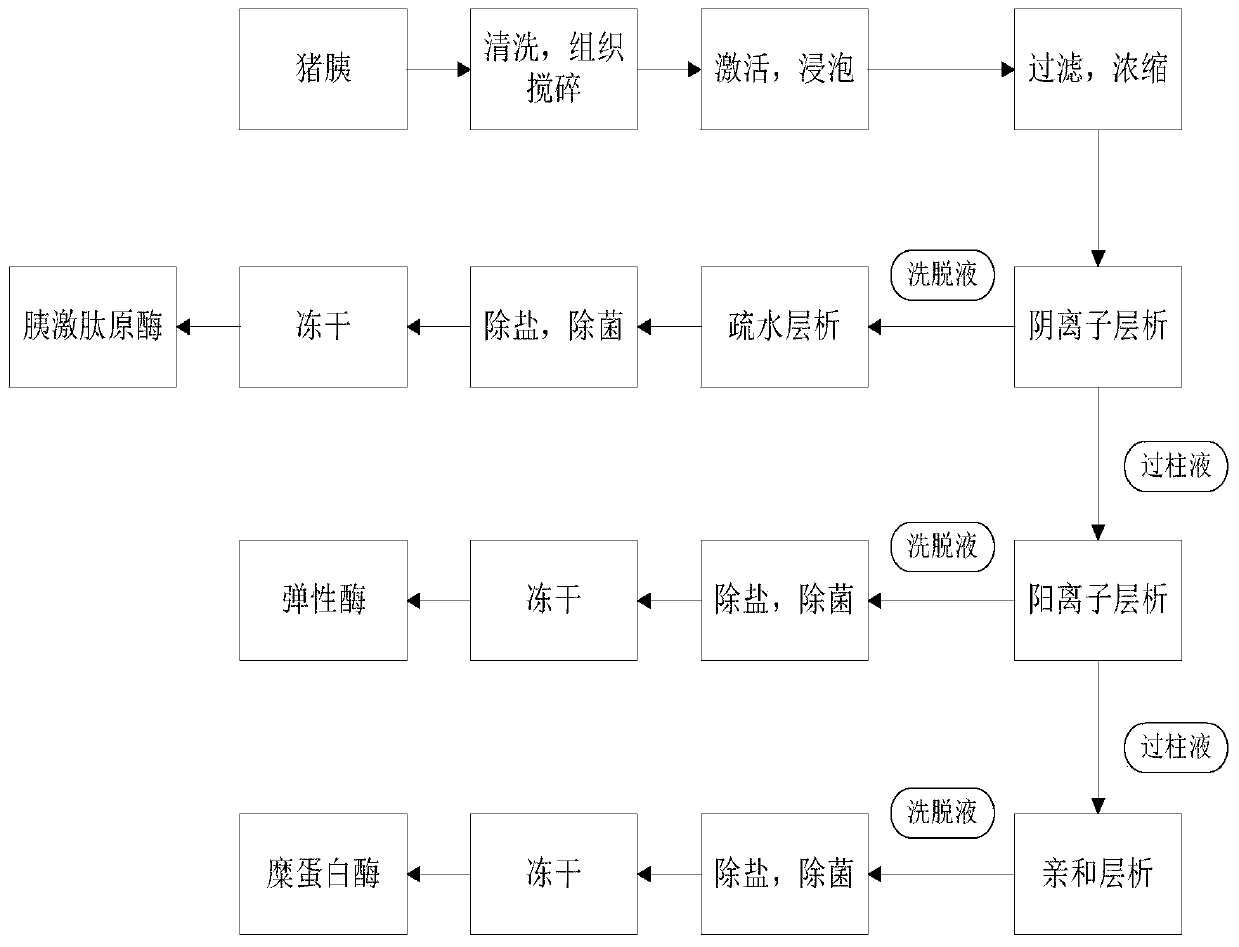
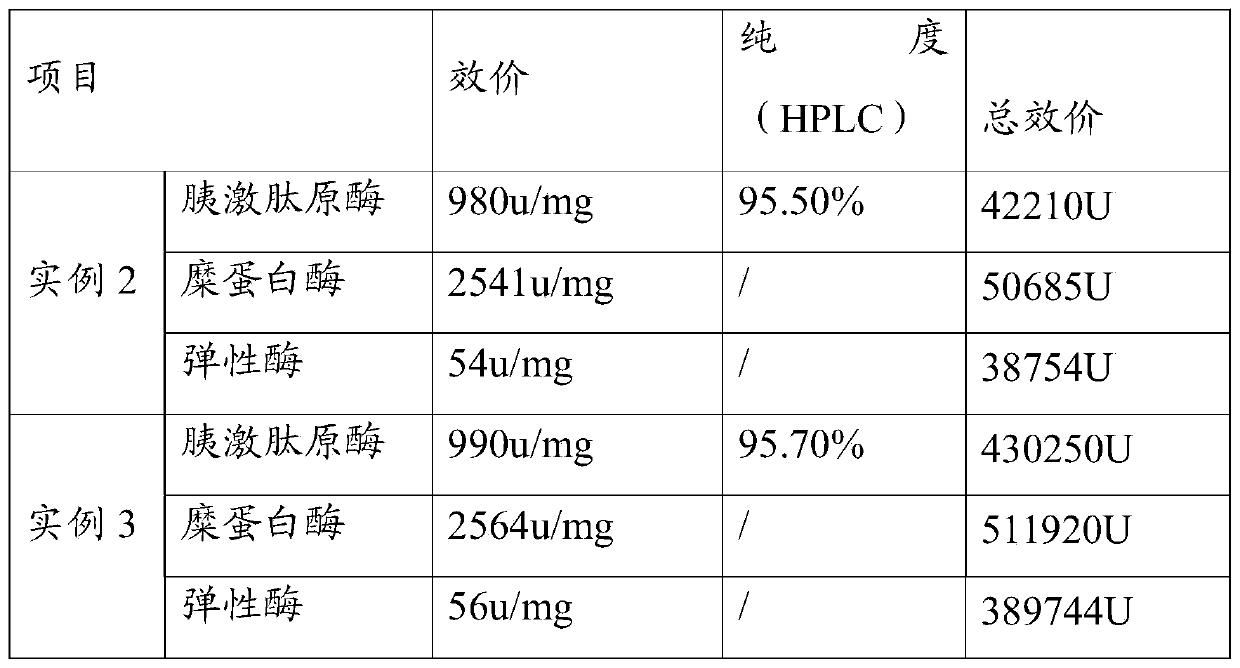
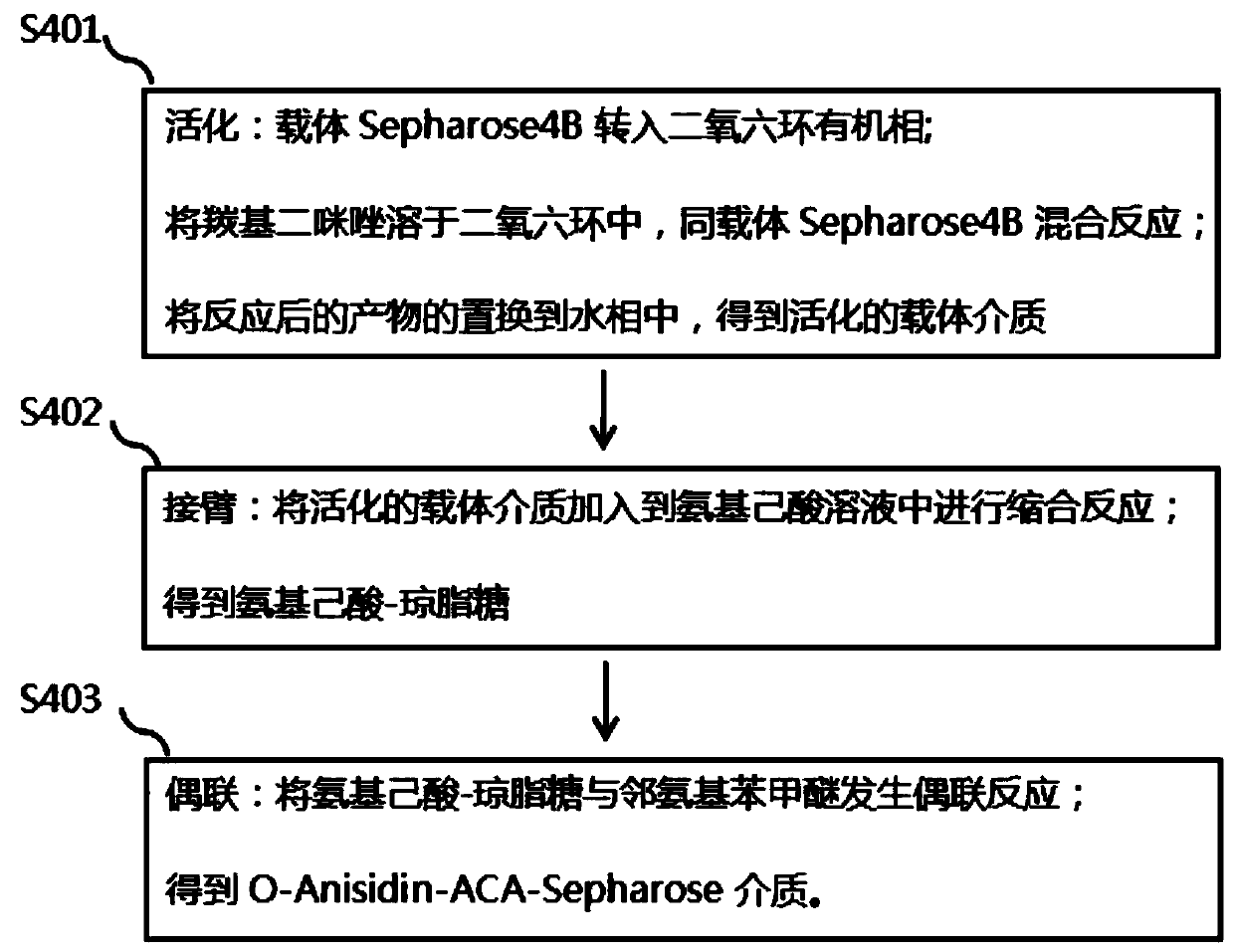
Process method for extracting three enzymes from animal pancreas
The invention discloses a process method for extracting three enzymes from animal pancreas. The process method comprises the following steps: by taking animal pancreas as a raw material, performing tissue crushing, activation, soaking, filtration, ultrafiltration, anion exchange chromatography and hydrophobic chromatography so as to obtain high-purity pancreatic kallidinogenase; performing cation-exchange chromatography on an anion exchange chromatography column-through fluid so as to obtain elastase; activating Sepharose, arming aminocaproic acid, coupling o-aminoanisole so as to obtain an O-Anisidin-ACA-Sepharose affinity adsorbent, and adsorbing a cation-exchange chromatography column-through fluid, so as to obtain high-purity chymotrypsin. By adopting the method, co-production of threeenzymes, namely pancreatic kallidinogenase, chymotrypsin and elastase, can be achieved, the operation is simple and convenient, the product purity is high, and resource waste can be reduced. Due to synthesis and use of an O-Anisidin-ACA-Sepharose medium, the production cost is reduced, and the product quality is improved.
Owner:江西浩然生物制药有限公司
Method of manufacturing 1-(2-methoxyphenyl)-3-naphthyl group-2-urea
ActiveCN101108813ADelay drug resistanceReduce the minimum inhibitory concentrationAntibacterial agentsOrganic chemistryRefluxAcetic acid
A preparation method of the bacterial drug-resistant inhibitor is provided, which is that the Beta-naphthamine is utilized as raw material and reacts with the urea to gain the N-(2-naphthol)- thiourea, and then reacts with the o-amino pheylmethyl ether to gain INF271, namely, 1-(2- methoxyphenyl)-3-naphthyl-2-thiourea. (1) The Beta-naphthamine and urea are added in water and are added with the diluted acid for 1h to 5h heating and reflux and are filtered after cooling to gain 1-(naphthol-2base)-thiourea; (2) 1-(naphthol-2base)-thiourea and the o-amino pheylmethyl ether mix with the diluted acid and are heated to 100 DEG C. to 108 DEG C., and then react for 1h to 5h to gain the 1-(2- methoxyphenyl)-3-naphthyl-2-thiourea after cooling and filtering. The diluted acid applied in the reaction can be sulphuric acid, hydrochloric acid and acetic acid, etc. the priority is hydrochloric acid.
Owner:PU LIKE BIO ENG
Quick drying type color fixing agent and preparation method thereof
The invention discloses a quick drying type color fixing agent. The quick drying type color fixing agent is prepared from the following components in percentage by weight: 15-25 parts of 2,5-diclorobenzic acid, 10-20 parts of sorbic acid, 8-13 parts of pinacolonelidinone, 18-26 parts of o-aminoanisole, 8-15 parts of 4,2-dinitroanisole, 6-12 parts of 2-amino-3,4-dyhydroxy diphenyl ketone, 7-13 parts of triethylene tetramine, 3-15 parts of alpha-alpha-diethoxybenzophenone, 10-18 parts of cyclohexanol and 50-80 parts of distilled water. By virtue of the color fixing agent, total time of a fabric color fixing process can be shortened, the color fixing efficiency is improved and the color fixing effect is good.
Owner:WUJIANG ZEWANG TEXTILE CO LTD
A kind of preparation method of environment-friendly CI disperse orange 29
ActiveCN104629493BThe method is simple and economicalConforms to EU standardsOrganic dyesDisperse dyeSlurry
Owner:浙江双冠染料有限公司
Preparation method of o-aminoanisole
InactiveCN103709051AReduce energy consumptionNo distillationOrganic compound preparationAmino-hyroxy compound preparationHydrogenO-Nitroanisole
The invention discloses a preparation method of o-aminoanisole. The preparation method comprises the following steps: adding 4000L of o-nitroanisole into a reduction reaction kettle by using a feeding pump; closing all valves of a reduction reactor; entirely replacing oxygen in the reaction kettle by nitrogen; turning on a stirring device of the reduction reactor after replacing; adding 15kg of a catalyst, heating to 60 DEG C and introducing 600m<3> of hydrogen; heating through reaction heat; keeping the temperature at 130 DEG C by using a jacket and a coiler to cool; controlling a reaction temperature at 60-150 DEG C and a reaction pressure within a range of 0.8-3.0MPa in a reaction process; reducing a temperature inside the kettle to 40 DEG C after the reaction; stopping stirring to settle the catalyst; filtering out the catalyst; sending o-aminoanisole to a layering device; standing for layering; and finally, rectifying to obtain refined o-aminoanisole. The preparation method disclosed by the invention has no need of distillation; the energy consumption of steam is reduced by more than 80%; the yield can be doubled.
Owner:于宝江
A kind of method utilizing meta-position oil to prepare m-chloroaniline
ActiveCN106883129BLow costHigh outputOrganic compound preparationAmino compound preparationSodium hydrosulfideAniline
The invention relates to a method for preparing m-chloroaniline by using meta-position oil, which belongs to the technical field of preparing m-chloroaniline, and specifically includes the steps of batching reaction, water washing, drying, three-stage vacuum distillation, crystallization, reduction, redistillation and the like. The present invention uses waste meta-oil produced by p- and o-nitrogen and industrial waste liquid sodium hydrosulfide as raw materials, utilizes methoxylation, crystallization and reduction to prepare m-chloroaniline, and generates by-products o-aminoanisole and p- Anisole, thereby realizing low cost, high output, compared with existing production technology, has obvious cost advantage, has higher economic benefit.
Owner:AZUREWAVE TEHNOLOGIES INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for synthesizing pyrazole [3,4-d] pyrimidine-4(5H)-ketone compounds Method for synthesizing pyrazole [3,4-d] pyrimidine-4(5H)-ketone compounds](https://images-eureka.patsnap.com/patent_img/33df1dce-a2f7-40ee-b292-1479c8dba829/s2007103042716c00011.PNG)
![Method for synthesizing pyrazole [3,4-d] pyrimidine-4(5H)-ketone compounds Method for synthesizing pyrazole [3,4-d] pyrimidine-4(5H)-ketone compounds](https://images-eureka.patsnap.com/patent_img/33df1dce-a2f7-40ee-b292-1479c8dba829/s2007103042716d00021.PNG)
![Method for synthesizing pyrazole [3,4-d] pyrimidine-4(5H)-ketone compounds Method for synthesizing pyrazole [3,4-d] pyrimidine-4(5H)-ketone compounds](https://images-eureka.patsnap.com/patent_img/33df1dce-a2f7-40ee-b292-1479c8dba829/s2007103042716d00031.PNG)