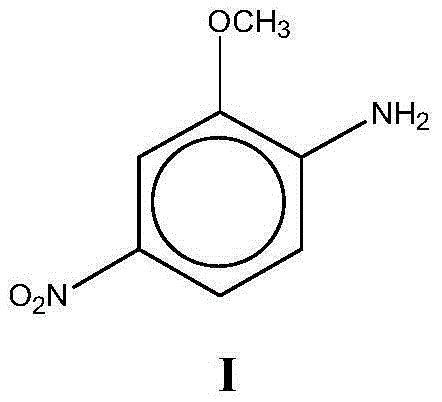
Preparation method for red base B
A red-based, methoxy-based technology, applied in the preparation of organic compounds, the preparation of aminohydroxy compounds, chemical instruments and methods, etc., can solve the problems of large amount of wastewater discharge and large proportion, and achieve less wastewater discharge and steric hindrance. Small, less by-product effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
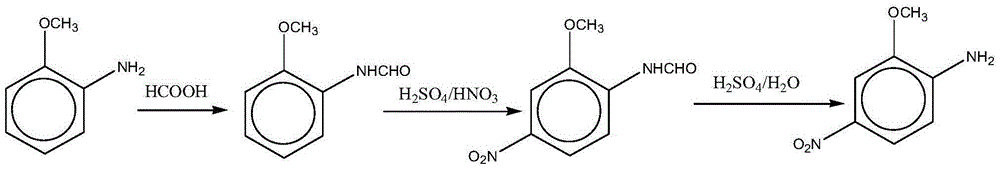
[0036] A clean preparation method for red base B, comprising the steps of:
[0037] (1) Preparation of o-carboxamido anisole: add 40g of anthranilate in a 500ml reaction vessel, add 350ml of toluene, add 20.9g of formic acid, start stirring, heat up to 100-102°C, and react for 3 hours , reclaim formic acid and toluene by distillation, add 300ml water to the residue, stir for 1 hour, leave standstill for 2 hours, filter to obtain o-carboxamide anisole, liquid chromatography analysis (area normalization method, the same below) product purity 96.13%, fold The hundred weight is 46.61g, and the yield is 95%.
[0038] (2) Preparation of 2-methoxy-4-nitrocarboxanilide: add 200ml of 98% concentrated sulfuric acid in a 500ml reaction vessel, start stirring, slowly add the o-carboxamidoanisole prepared in step (1), At a temperature of 35°C, slowly add 25.2 grams of 98% nitric acid dropwise, control the reaction temperature at 35-45°C, and the dropwise addition time is about 1.5 hours. ...
Embodiment 2
[0041] This example provides a clean preparation method for red base B, which is basically the same as Example 1. The difference is: in the first step in Example 1, 350ml of chlorobenzene was used as the solvent for the acylation reaction of o-formamide anisole, which was used to prepare o-formamide anisole, and o-formamide anisole was obtained. The product had a purity of 96.2%. The hundred weight is 46.72g, and the yield is 95.2%.
Embodiment 3
[0043] This example provides a clean preparation method for red base B, which is basically the same as Example 1. The difference is: the filtrate produced during the second step in Example 1 to prepare 2-methoxy-4-nitrocarboxanilide is denitrated and concentrated to a solution with a mass concentration of sulfuric acid of 89% and a solution with a mass concentration of 98%. Concentrated sulfuric acid is mixed in a volume ratio of 4:1 to form a mixed solution, which is used as a sulfuric acid solution for the preparation of 2-methoxy-4-nitroformanilide to obtain 2-methoxy-4 -Nitroformanilide was converted into 56.43g, and the yield was 93.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com