Electrode catalyst for fuel cell and fuel cell
A technology for electrode catalysts and fuel cells, which is applied in the direction of fuel cells, battery electrodes, solid electrolyte fuel cells, etc., can solve the problems of inability to obtain, and achieve the effects of improving high output, reducing size, and improving battery voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
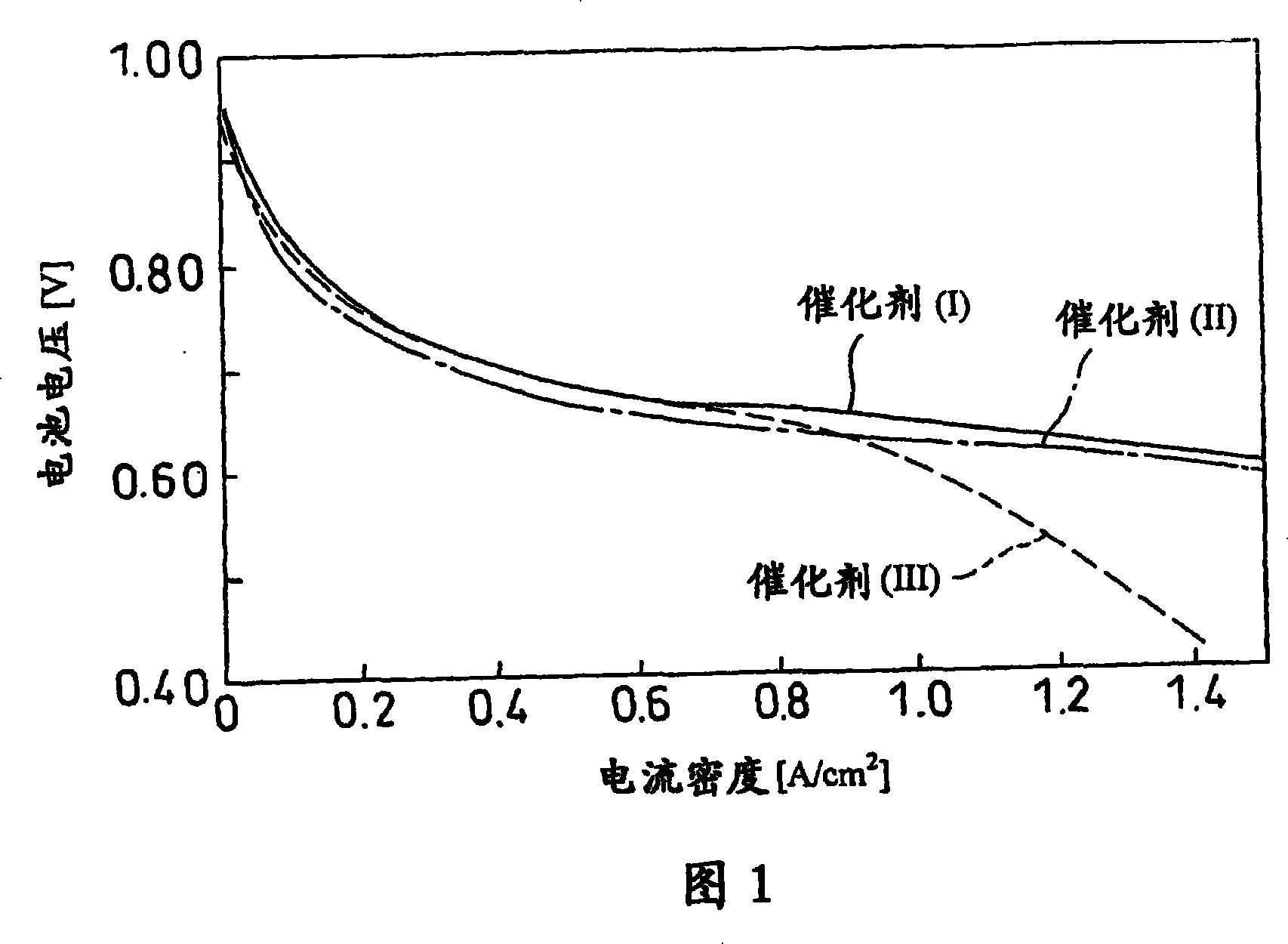
[0034] will have approximately 1000m 2 A commercially available carbon black powder having a specific surface area per g (50 grams) was added to 0.5 liter of pure water and allowed to disperse therein. A chloroplatinic acid solution containing 5.0 g of platinum was added dropwise to the obtained diffusion solution, and mixed well with carbon. Then, the solution was neutralized with an ammonia solution, followed by filtration. Next, the above-prepared cake was evenly dispersed in one liter of pure water again. A diffusion solution prepared by dissolving cobalt nitride containing 0.5 g of cobalt in 0.1 liter of pure water was added dropwise to the solution. The resulting solution was neutralized with an ammonia solution, followed by filtration. The resulting cake was vacuum dried at 100°C for 10 hours. Afterwards, the obtained product was alloyed at 600° C. for 6 hours under an argon atmosphere in an electric furnace. The catalyst prepared after alloying was identified as C...
example 2
[0039] Catalyst A (10 grams) was stirred in 1 liter of nitric acid solution (3 mol / liter) and then kept in solution at 90° C. for 1 hour, followed by filtration. The resulting cake was vacuum dried at 100°C for 10 hours. Thereafter, the obtained product was reduced at 100° C. for 1 hour in a hydrogen atmosphere of an electric furnace, thereby obtaining a catalyst powder (II).
[0040] As in the case of Example 1, the physical properties of the catalysts were determined. The measured catalyst particle size is 3.7 nanometers, the amount of basic surface functional groups is 62meq, the pH value in water is 6.8, and the specific surface area is 378m 2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com