Application of recombinant adenovirus in producing antineoplastic medicine
A recombinant adenovirus and anti-tumor drug technology, applied in the field of biology, can solve the problems of difficult to achieve effective concentration in vitro, unable to exert drug effect, insufficient drug intake, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Construction and preparation of recombinant adenovirus (Ad-WWOX)
[0036] (1) Construction of recombinant adenovirus
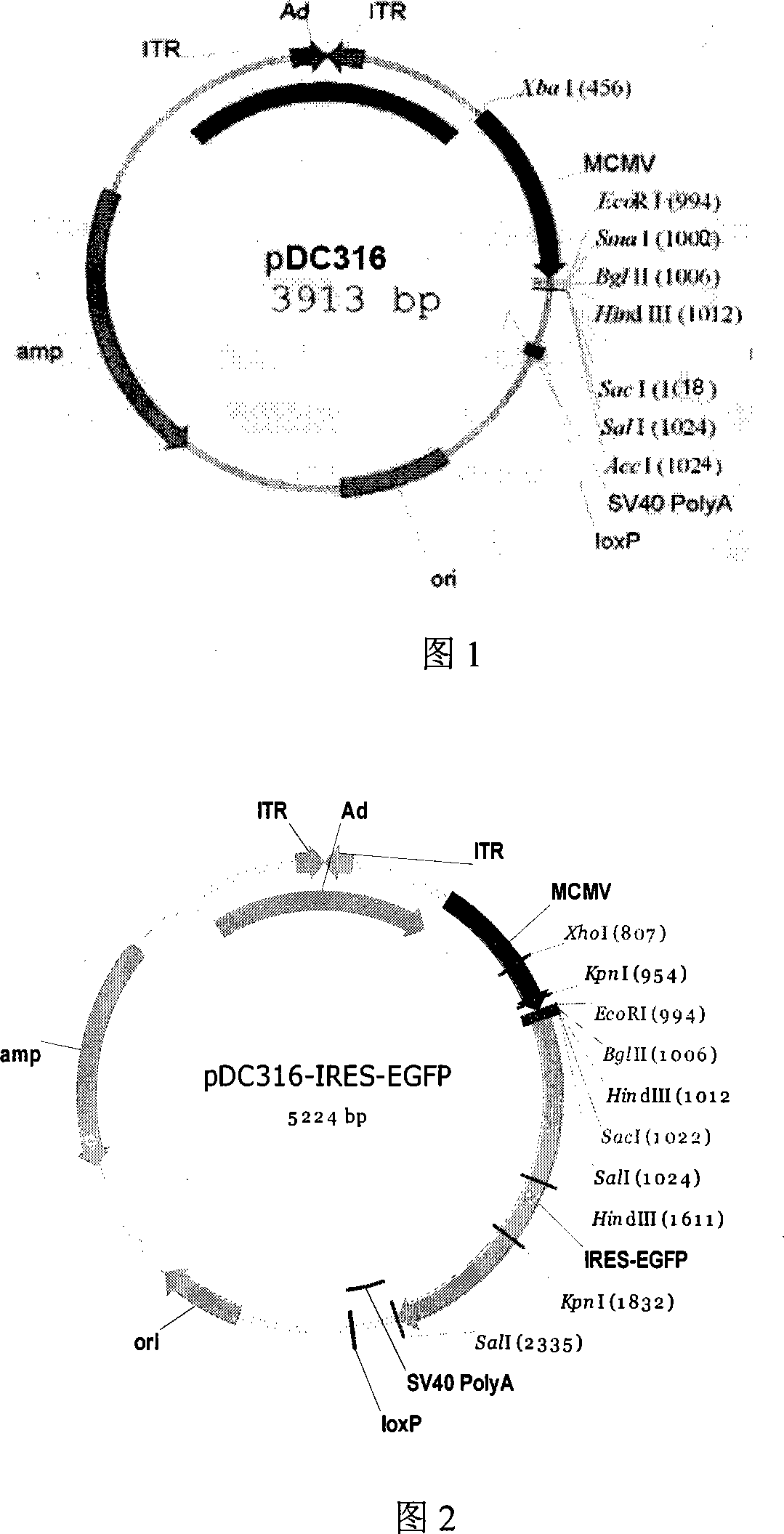
[0037] Replication-deficient adenovirus AdMax adenovirus packaging system (product of MicrobixBiosystems, Canada, the map of the shuttle plasmid—pDC316 plasmid is shown in Figure 1, and the backbone plasmid—pBHGlox_E1, 3Cre.)
[0038] 293 cells are products of MICROBIX BIOSYSTEMS, Canada.
[0039] 1. Construction of pDC316-IRES-EGFP plasmid
[0040] 1.1 Amplified sequence IRES-EGFP from plasmid pIRES-EGFP
[0041] Primers were designed (primer design software: oligo6.0), the sequence IRES-EGFP was amplified from the plasmid pIRES-EGFP (purchased from clontech company), and the plasmid pDC316-IRES-EGFP was constructed.
[0042] The primer sequences are:
[0043] IRES up 5'-GCCAGTCGACACCGCATCGAGCTGA-3'
[0044] IRES down 5'-CTGCCAAGTTGCTCGAAGTCGACT-3'
[0045] Using primers IRES up and IRES down to amplify fragment IRES-EGFP from pIRES...
Embodiment 2
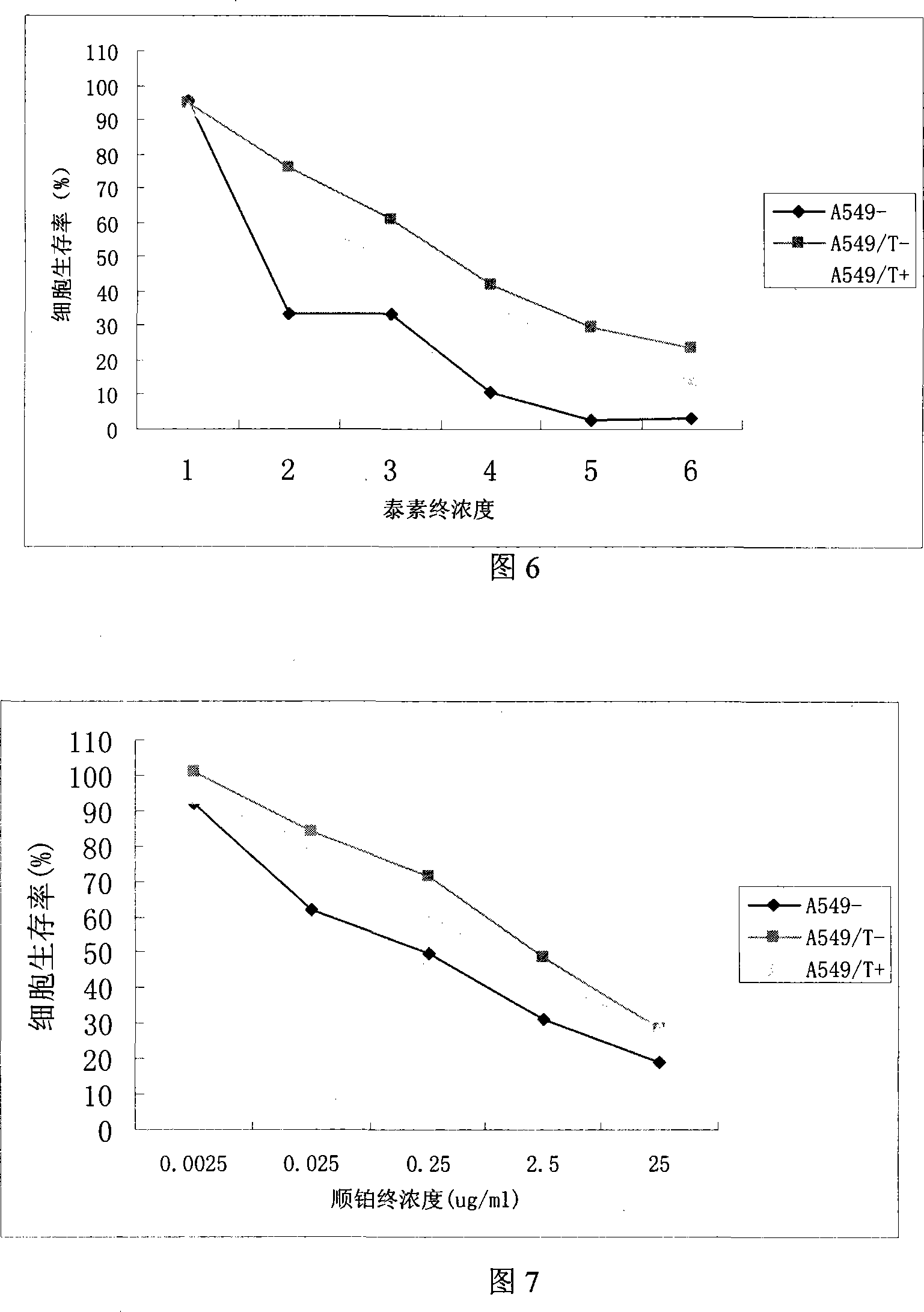
[0156] Example 2: Experimental Study of Recombinant Adenovirus Mediated Human WWOX Gene on Drug Resistance Gene Therapy of Human Lung Adenocarcinoma Cells
[0157] (1) Detection of drug resistance gene therapy in lung adenocarcinoma drug-resistant cells (in vitro drug sensitivity test-CCK-8 method)
[0158]A549 / T+ group, A549 / T-group and A549-group grew up to 80% infected with Ad (MOI: 50), collected cells in 48 hours; added to 96-well culture plate (100ul complete medium+8000 cells / well), Incubate for 12 hours, add different concentrations of CDDP (Australian Keding Pharmaceutical Co., Ltd.) or TAX (three replicate wells for each well); incubate in a 37°C incubator for 72 hours, suck up the disposable infusion needle connected to the vacuum pump to measure Well culture solution, add complete medium (premixed 5ul CCK-8+100ul medium containing 10% fetal bovine / well), cultivate for 3.5 hours; measure the absorbance at 450nm (reference wavelength 650nm), calculate the cell surviv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com