Pharmaceutical composition containing human urine kininogenase for treating cerebral infarction
A technique for urokininogenase and composition, which is applied in the fields of biochemistry and medicinal chemistry, can solve the problems such as unreported research on human urokininogenase, and achieve the effects of less side effects, prevention of cerebral infarction, and reduced side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
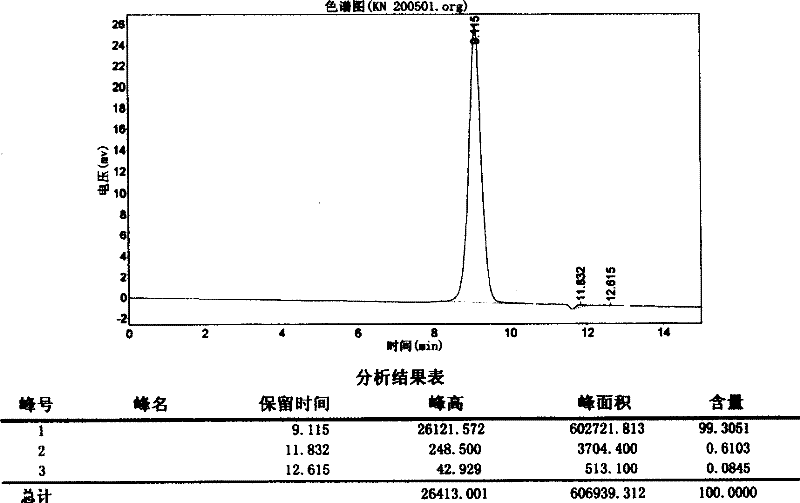
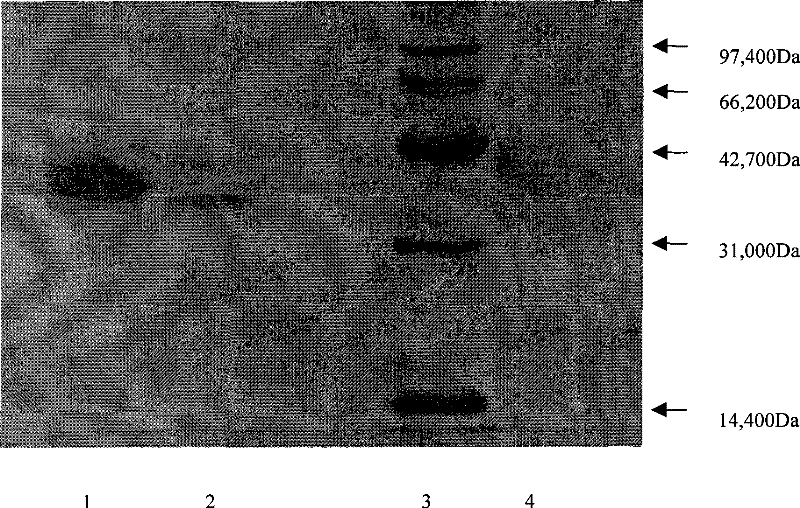
[0042] Embodiment 1 SDS-PAGE electrophoresis assay shows the preparation of the human urinary kininogenase of single band
[0043] It is prepared by the following steps (benzaprimidine affinity chromatography):
[0044] 1. Pump 10 tons of fresh male urine into the stirring tank, adjust the pH to 4.5-5.5, add 300kg of deacetylated chitin for adsorption, and adjust the pH to 7.0-9.0 with 10% ammonium sulfate solution to elute; the eluent Use diatomaceous earth as the filter medium, perform suction filtration, drain the salting-out solution completely, and 1 kg of the drained product is the crude product of human urinary kallikreinase;
[0045] 2. Pour 150kg of crude human urinary kininogenase into a stirring tank, add 750L of 0.15M EDTA solution to dissolve, adjust the pH to 9.0, and stir until the crude product is completely dissolved; centrifuge at 3000 rpm for 20 minutes, take the Clear, filter the membrane of 0.45 μm, ultrafiltration obtains ultrafiltrate 45L, therefrom get...
Embodiment 2
[0058] Example 2 Determination of the structure of human urinary kininogenase
[0059] Amino Acid Composition Analysis of Human Urinary Kallikrein:
[0060] 1. Instrument name and model: Hitachi 835-50 high-speed amino acid analyzer
[0061] 2. Sample preparation:
[0062] Accurately measure the sample into the hydrolysis tube, add 6mol / L hydrochloric acid, hydrolyze at 110°C for 24 hours, cool to constant volume, filter, evaporate to remove excess hydrochloric acid, constant volume with 0.02mol / L, and analyze on the machine.
[0063] 3. Determination conditions:
[0064] Ion exchange column: 2.6×150mm; resin specification: No.2619; column temperature: 53°C; pump flow rate: 0.225ml / min; pump pressure: 90kg / cm2, eluent IPH—1, 2, 3, 4; analysis Time: 72min; injection volume: 50μl.
[0065] 4. Measurement results: basically consistent with the theoretically derived values (Table 3).
[0066] Table 3 Analysis results of amino acid composition
[0067]
[0068]
[006...
Embodiment 3
[0071] Example 3 Study on Enzymatic Properties of Human Urinary Kinikreinogenase
[0072] Take 2 small test tubes (2×20cm), add 0.2ml of human urinary kininogenase solution to each, add 4.0ml of 0.2mol / L Tris buffer solution (pH8.0), mix well, and incubate at 37°C for 5 minutes. Add 0.4ml of 50% acetic acid solution to the first tube, add 0.4ml of substrate solution (S-2266 (HD-Val-Leu-Arg-PnA·2HCl), 1.5mM) to the second tube, mix well, and React in a water bath at ±0.5°C for 15 minutes, then add 0.4ml of substrate solution to the first tube, add 0.4ml of 50% acetic acid solution to the second tube, measure at a wavelength of 405nm, and use the first tube as a blank to measure the absorption value A, A value should be controlled between 0.1 and 0.2. Substitute the value of A into the following calculation:
[0073] PNA U / ml=173.6×A×T / 1000 (T is the dilution factor)
[0074] 1PNA U (p-nitroaniline unit): equivalent to human urinary kininogenase when H-D-Val-Leu-Arg-p-nitroni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com