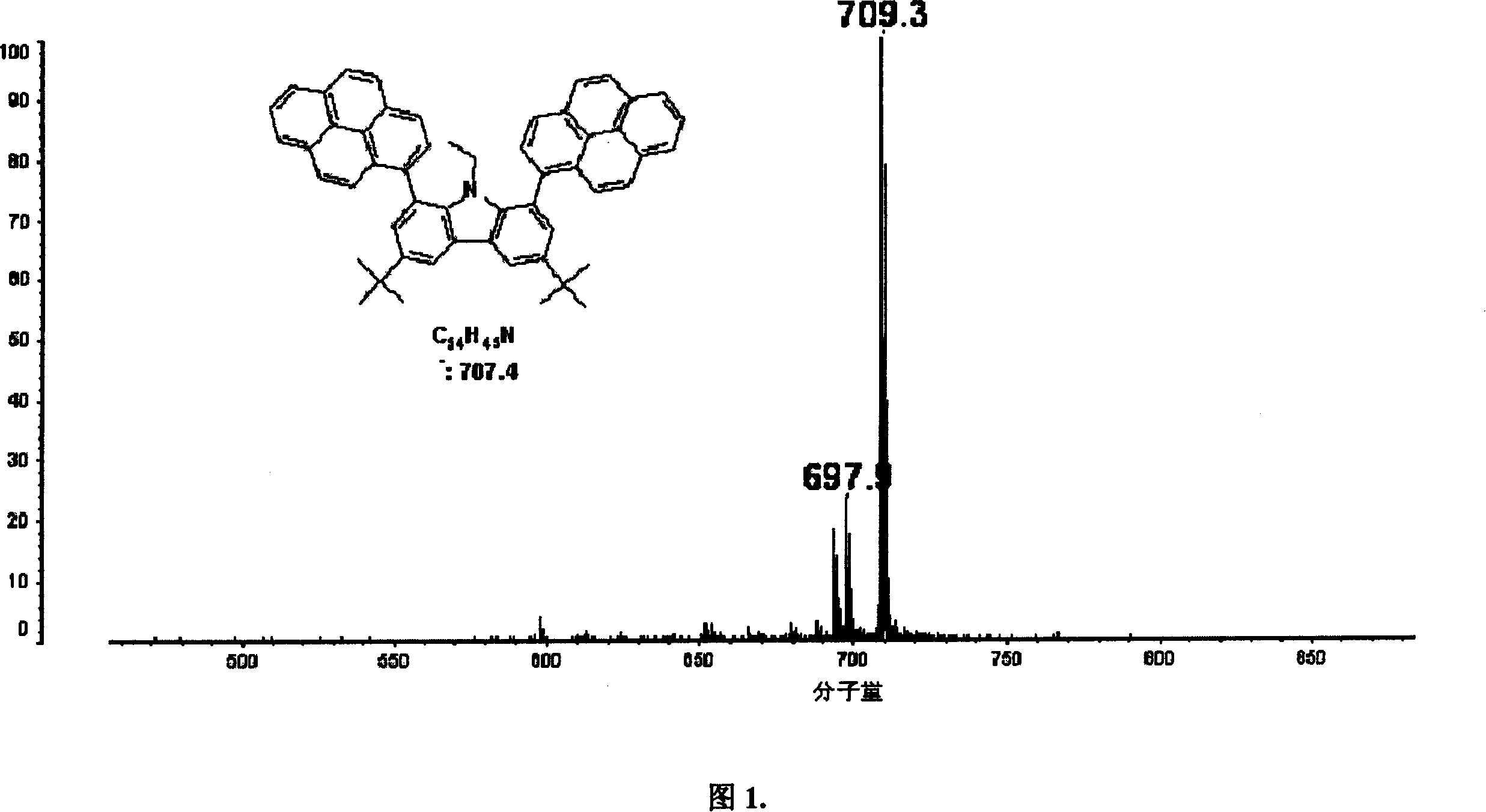
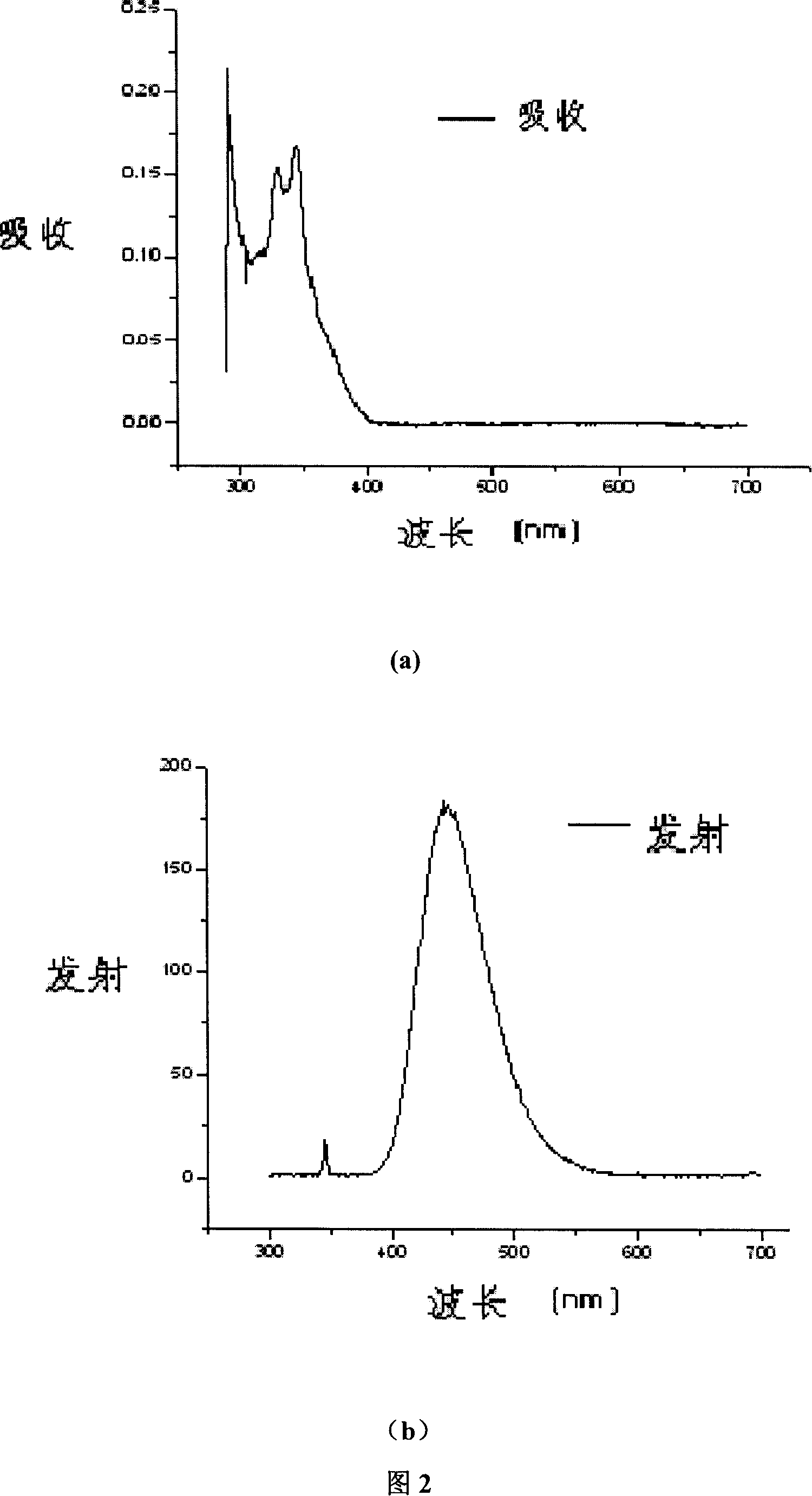
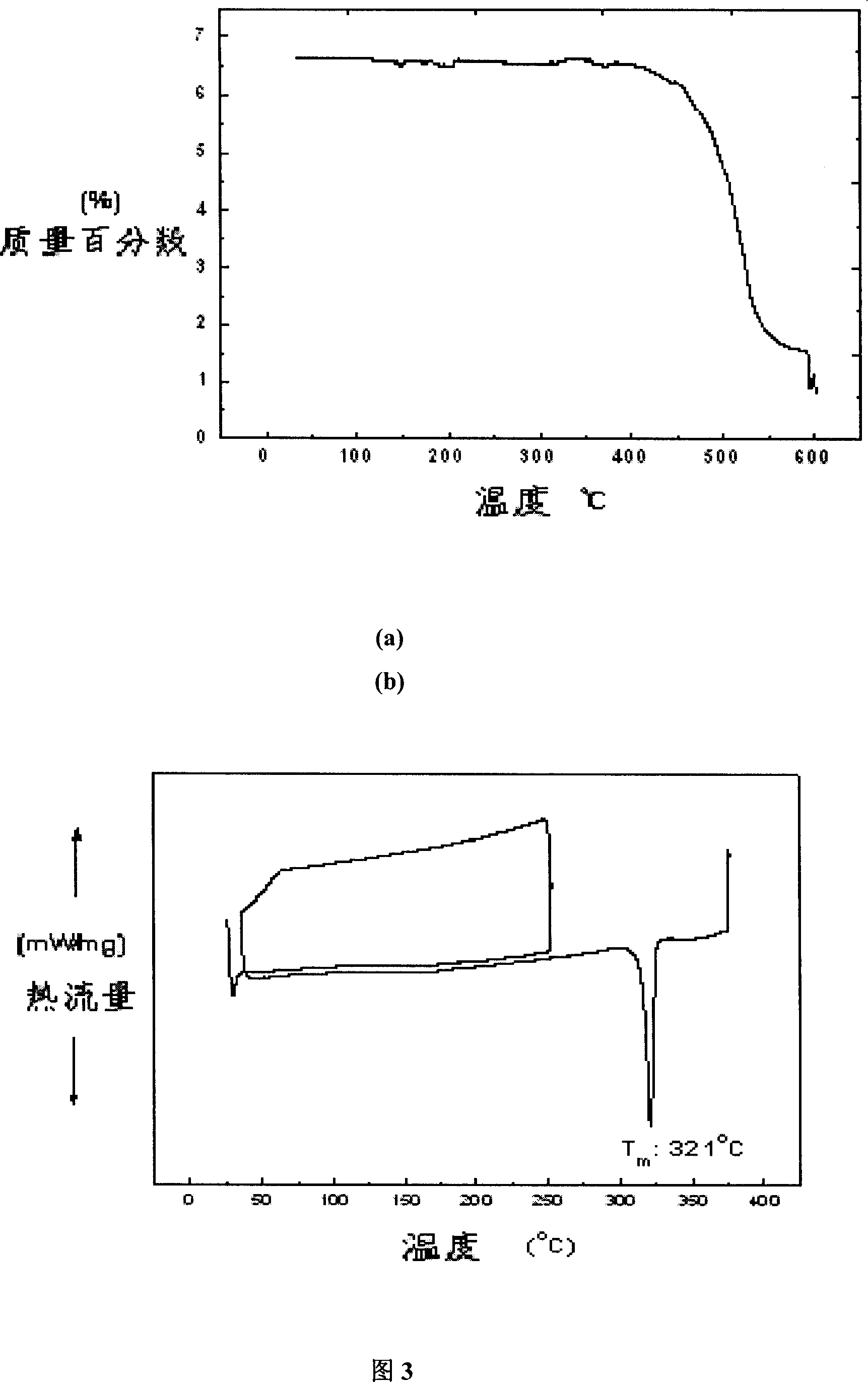
Organic semiconductor material containing carbazole unit and synthesis
A technology of organic semiconductors and carbazole units, which is applied in the field of organic semiconductor materials containing carbazole units and its preparation, can solve the problems of limiting the application range of carbazole materials, achieve good evaporation performance, large modification space, and flexible routes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, compound material containing carbazole and pyrene:
[0045] 9-Ethyl-carbazole
[0046] 9-ethyl-9H-carbazole
[0047] Mix 5g carbazole, 3g KOH, and 30ml DMSO in a three-necked flask, add 4ml bromoethane dropwise under the condition of 85 degrees, stir for 3 hours, add 100ml water after cooling and mix, and leave a light yellow solid to precipitate, filter, After drying and recrystallization from absolute ethanol, the product was obtained as white needle crystals (yield 90%).
[0048] GC-MS (EI-m / z): 195 (M + ).
[0049] 3,6-di-tert-butyl-9-ethyl-carbazole
[0050] 3,6-di-tert-butyl-9-ethyl-9H-carbazole
[0051] Take 9-ethyl-carbazole (20mmol), 100mlCH 2 NO 3 , and zinc chloride (8.1g, 60mmol) were mixed in a three-neck flask, under nitrogen conditions, tert-butyl chloride (6.5ml, 60mmol) was added dropwise in the reaction flask, and after 5 hours of normal temperature reaction, 200ml The reaction was quenched with water, extracted with dichlorometha...
Embodiment 2
[0068] Embodiment 2, compound material containing carbazole and anthracene:
[0069] 10-bromoanthracene
[0070] 10-bromoanthracene
[0071] Anthracene (12.5mmol) was dissolved in DMF (17.42mL), and NBS (2.046g, 11.5mmol) was dissolved in DMF (21.78mL), and the mixture was added dropwise under ice water. After the addition, it was stirred at room temperature for 24 hours. Diluted with water, extracted with ether, dried and rotary evaporated, and purified with petroleum ether silica gel column to obtain a white solid (yield 88%).
[0072] GC-MS (EI-m / z): 280 (M + ).
[0073] 10-Anthraceneboronic acid
[0074] anthracen-10-yl-10-boronic acid
[0075]First, take 10-bromoanthracene (2.53mmol) and put it into a 250mL two-necked flask. The flask has been heat-dried and airtight, and then evacuated three times with nitrogen. Then, the reaction apparatus was put into a low temperature bath of -78°C generated by dry ice and acetone, and freshly distilled tetrahydrofuran (20 mL) w...
Embodiment 3
[0080] Embodiment 3, compound material containing carbazole and benzanthracene:
[0081] 10-Bromo-9-phenylanthracene
[0082] 10-bromo-9-phenylanthracene
[0083] Dissolve 9-phenylanthracene (1.5g, 5.9mmol) in acetic acid (80mL), heat to 65°C under nitrogen atmosphere, and add bromine (1.04g, 6.5mmol) dissolved in acetic acid (20mL) dropwise into the reaction flask. After the dropwise addition, the reaction was allowed to return to room temperature, crystals were precipitated, and filtered to obtain a yellow solid (92% yield).
[0084] GC-MS (EI-m / z): 280 (M + ), mp154°C.
[0085] 9-Phenylanthracene-10-yl-10-boronic acid
[0086] 9-phenylanthracen-10-yl-10-boronic acid
[0087] First, take 10-bromo-9-phenylanthracene (2.53mmol) and put it into a 250ml two-necked flask. The flask has been heated, dried and airtight, and then vacuumed three times with nitrogen. Oxygen device for processing use. Then, the reaction apparatus was put into a low temperature bath of -78°C gen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com