On-site hydrogen producing method and on-site hydrogen producing device
An on-site, reforming hydrogen production technology, applied in chemical instruments and methods, hydrogen, inorganic chemistry, etc., can solve the problems of hydrogen permeation rate and hydrogen selectivity decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] This embodiment is used to illustrate the on-site hydrogen production method and on-site hydrogen production device provided by the present invention.
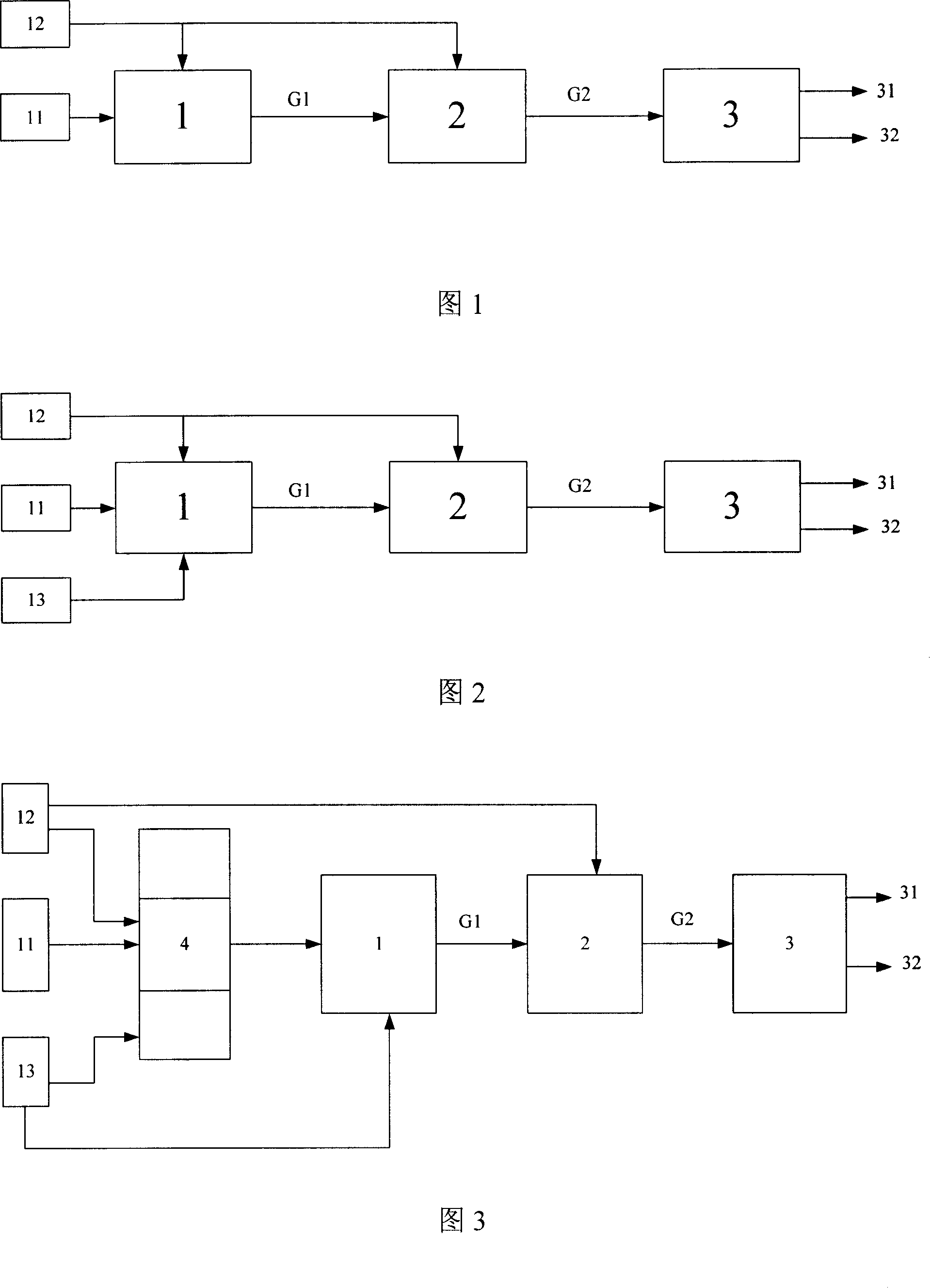
[0048] An on-site hydrogen production device as shown in Figure 3 was prepared.
[0049] Wherein, the carbon-containing raw material supply device 11 and the water supply device 12 are all rectangular parallelepiped storage tanks of 150 mm * 150 mm * 200 mm made of stainless steel.
[0050] The oxygen-containing gas supplier 13 is a gas bottle filled with compressed air; the reforming hydrogen production reactor 1 is a 1.4-liter tubular reactor made of 0.3 mm thick stainless steel 316.
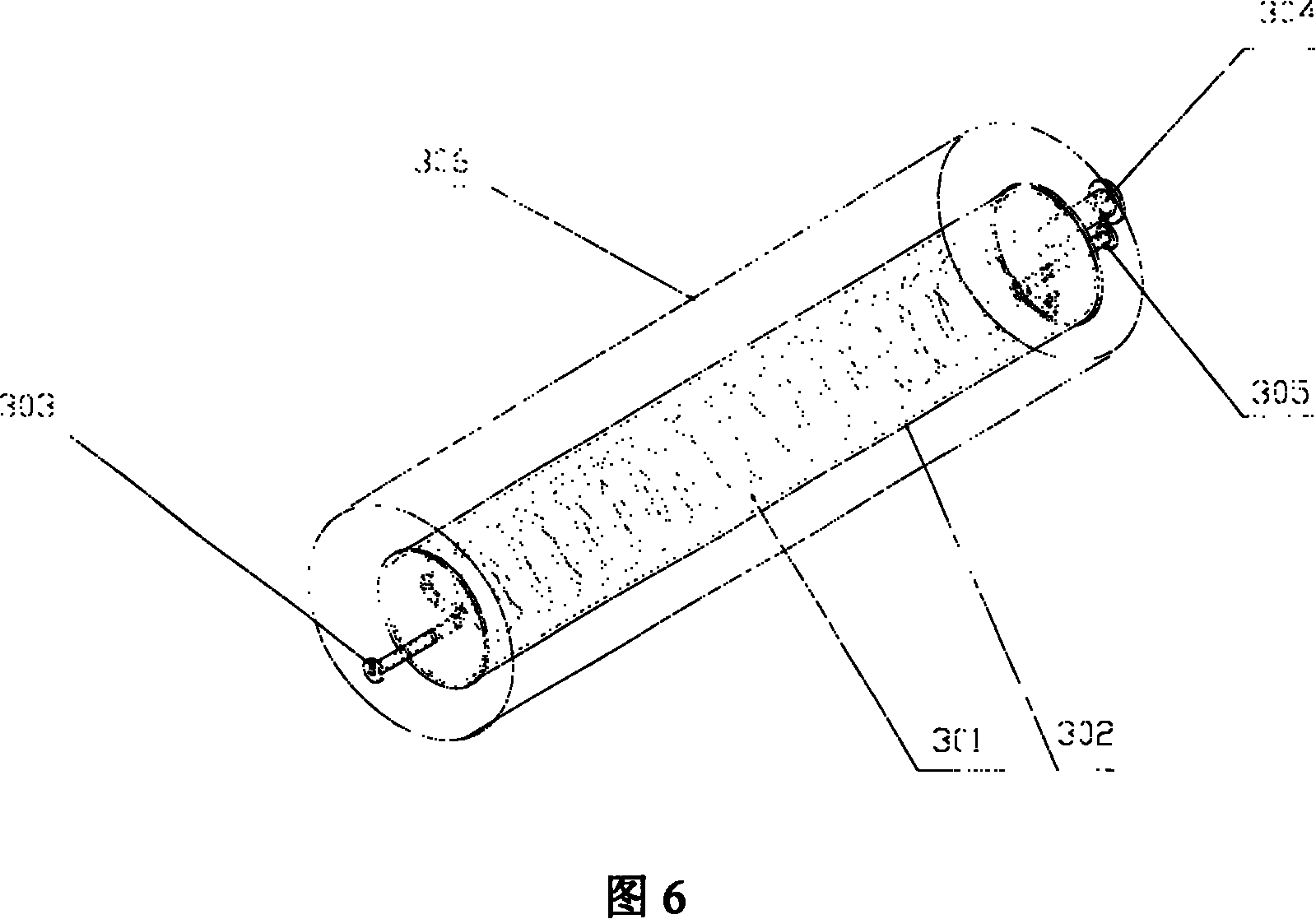
[0051] The structure of the hydrogen separator 3 is shown in Figure 6, the housing 306 and the hydrogen collection chamber 302 are tubular containers made of stainless steel 316, the length of the housing 306 is 20 centimeters, and the inner diameter is 2.6 centimeters, The hydrogen collection chamber 302 has a length of 10 cm and an i...
Embodiment 2
[0058] This embodiment is used to illustrate the on-site hydrogen production method and on-site hydrogen production device provided by the present invention.
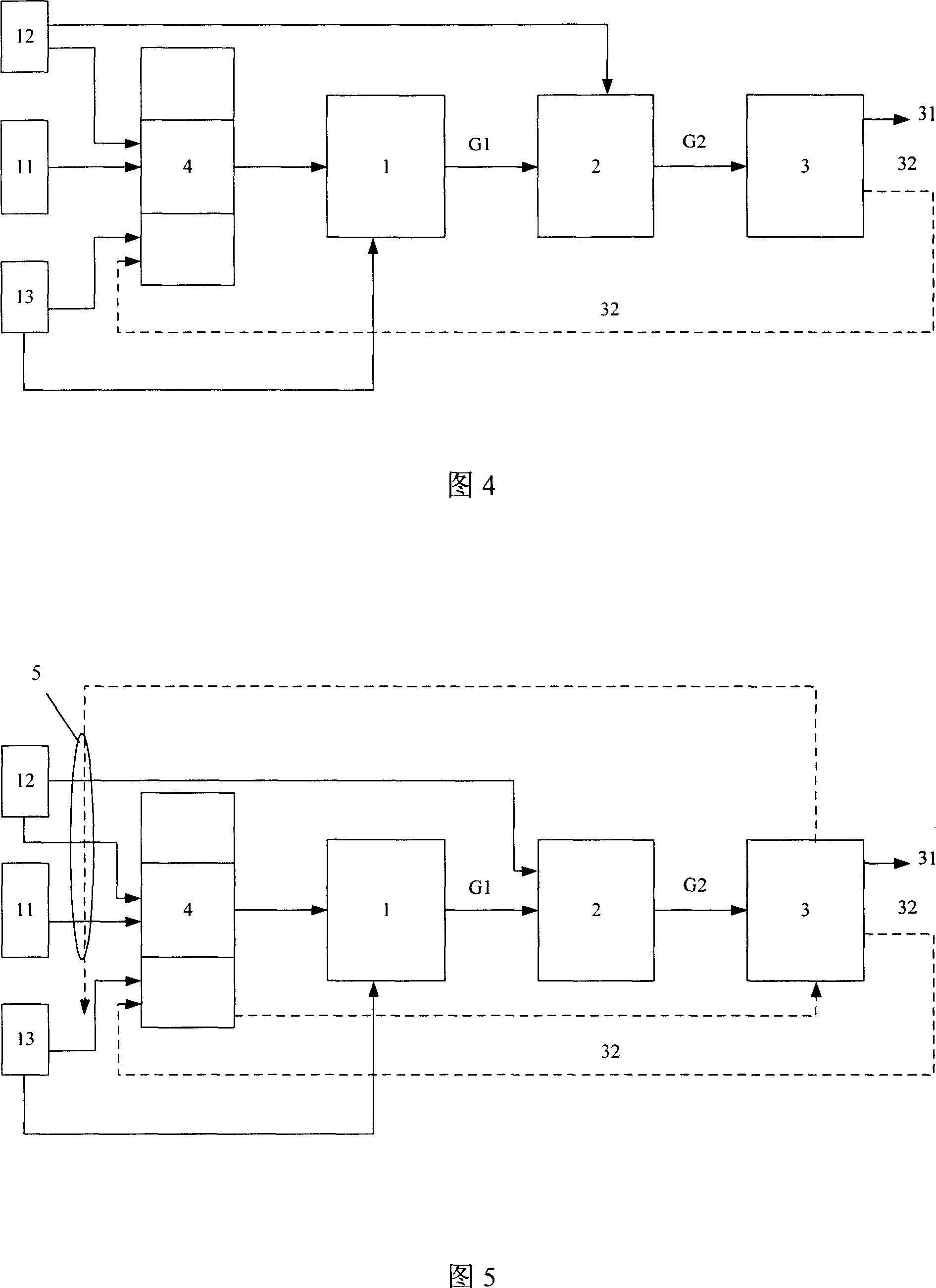
[0059] Adopt the same device, material and connection method as in Example 1 to prepare the on-site hydrogen production device as shown in Figure 3, the difference is that the catalyst in the reforming hydrogen production reactor 1 is a Cu-Zn-La alloy (Cu, Zn The molar ratio with La is 1:0.4:0.4) (Selectra (TM) catalyst produced by Yingge Chemical Company), supported on Al 2 o 3 On the coating, the coating density is 30 g / cm2, Al 2 o 3 The coating is coated on a 400-mesh cordierite honeycomb carrier, and the coating thickness is 20 microns; the water conversion catalyst is supported on Al 2 o 3 Pd-Cu alloy on the coating (the molar ratio of Pd and Cu is 1: 0.5) (Selectra (TM) catalyst produced by Yingge Chemical Company), coating density 20 grams / square centimeter, Al 2 o 3 Coated on a 400-mesh FeCrAl alloy honeyc...
Embodiment 3
[0062] This embodiment is used to illustrate the on-site hydrogen production method and on-site hydrogen production device provided by the present invention.
[0063] Adopt the same device, material and connection method as in Example 1 to prepare the on-site hydrogen production device as shown in Figure 3, the difference is that the catalyst in the reforming hydrogen production reactor 1 is a Pd-Ce alloy (the mole of Pd and Ce The ratio is 1:0.2) (Selectra (TM) catalyst produced by Yingge Chemical Company), supported on Al 2 o 3On the coating, the coating density is 30 g / cm2, Al 2 o 3 The coating is coated on a 400-mesh cordierite honeycomb carrier, and the coating thickness is 15 microns; the water conversion catalyst is supported on Al 2 o 3 Pd-Cu-Cr alloy on the coating (the molar ratio of Pd, Cu and Cr is 1: 0.5: 0.2) (Selectra (TM) catalyst produced by Yingge Chemical Company), coating density 20 grams / square centimeter, Al 2 o 3 Coated on a 400-mesh FeCrAl alloy h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com