Standard reagent kit for mass spectrometry clinical diagnosis and the method
A technology for standard materials and diagnostic reagents, which is applied in the field of protein detection and can solve the problem of lack of clinical mass spectrometry diagnostic reagents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Standardized quality control serum (plasma) preparation of embodiment 1 mass spectrometry
[0055] (1) Experimental method
[0056] 1. Materials
[0057] Specimen source: Standardized quality control serum (plasma) preparation for mass spectrometry. The definitions meet the following criteria. The blood donors are half male and half male, and the blood type is O; the age is 18-30 years old; ethnicity, Han. The biochemical indicators were normal, including: total cholesterol, triglycerides, fasting blood sugar, hepatitis B surface antigen, liver function test, kidney function test; no family history of genetic diseases; no history of major infectious diseases. Women without pregnancy, men with no history of smoking. Fresh blood was drawn from 10 healthy subjects with blood type O (half male and half female), and centrifuged at 4°C immediately after the blood coagulated, at 10,000rpm, 4°C for 5min; sample processing and storage. Take 10 μl of mixed serum (slurry), dilu...
Embodiment 2
[0062] Example 2 Analysis of serum (plasma) proteome has good reproducibility
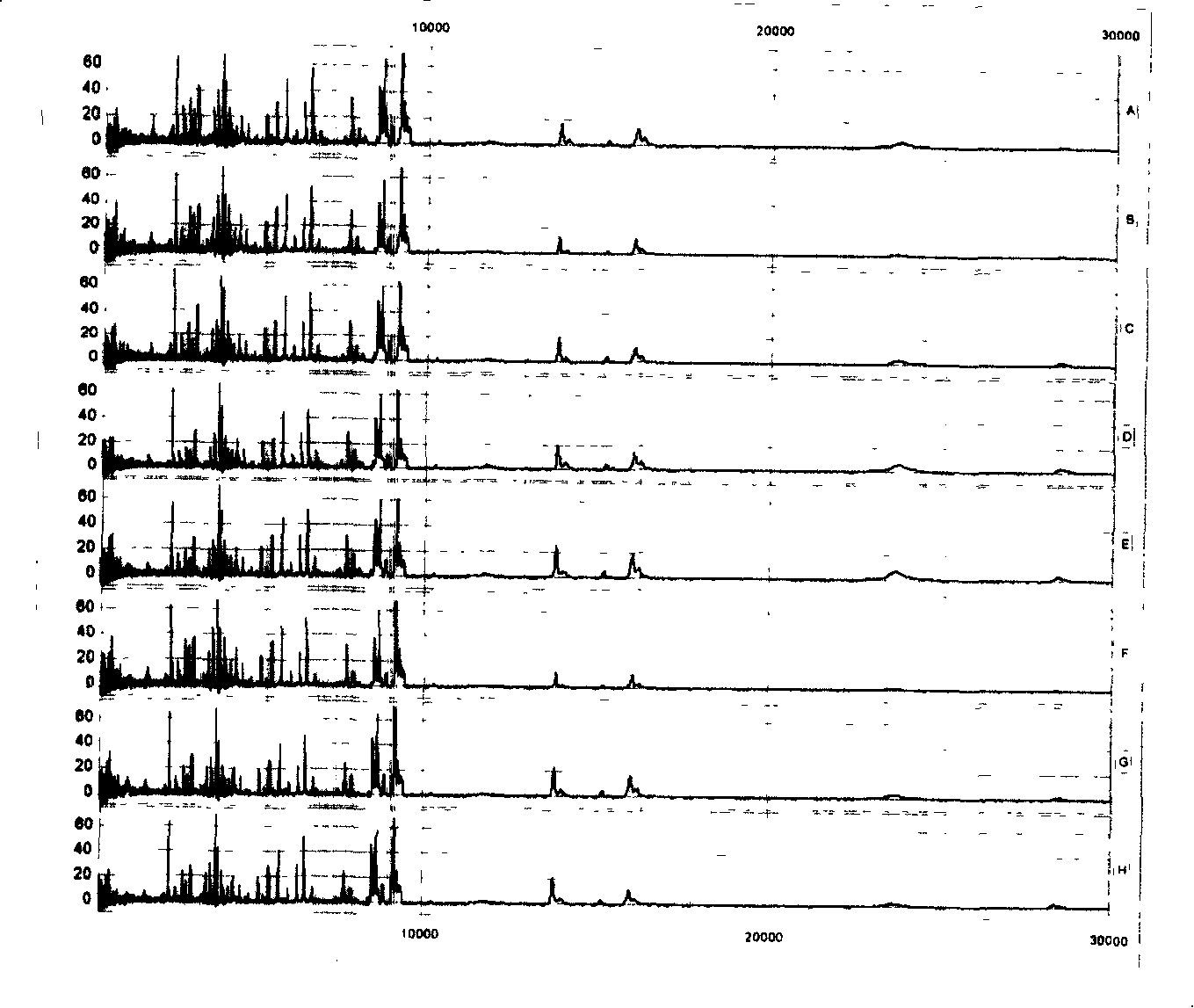
[0063] The above-mentioned mixed serum (serum) was spotted on 8 spots on the same chip for repeatability detection, and the peptides and low molecular weight proteomes in the serum (serum) were analyzed. like figure 1 As shown, among the 79 main peaks defined and analyzed, the coefficients of variation (CV) of the peak intensities are all below 10%, indicating that the analysis of the proteome by this method has good repeatability.
Embodiment 3
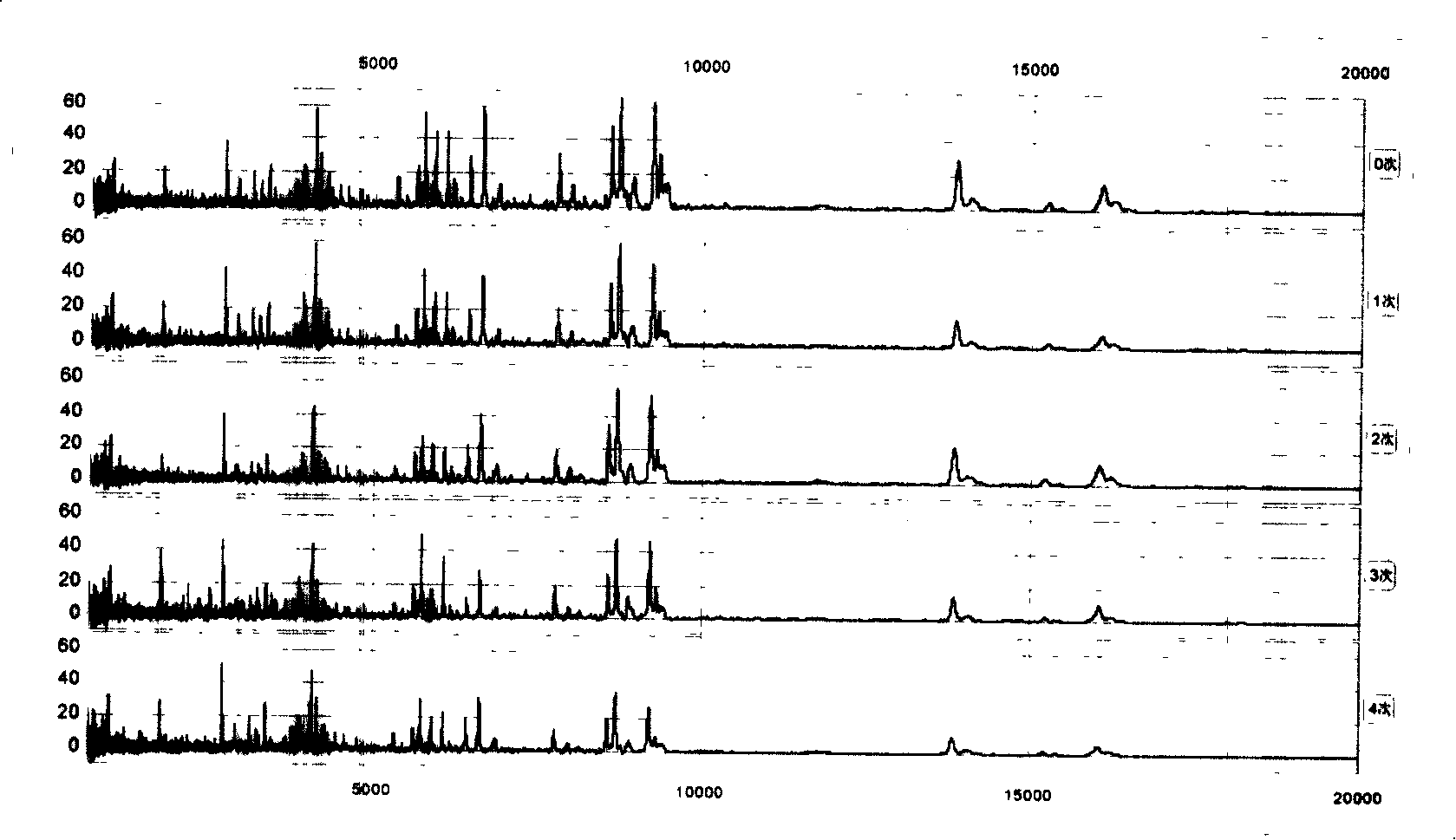
[0064] Example 3 Effects of different storage conditions of serum (slurry) on the results of the proteome
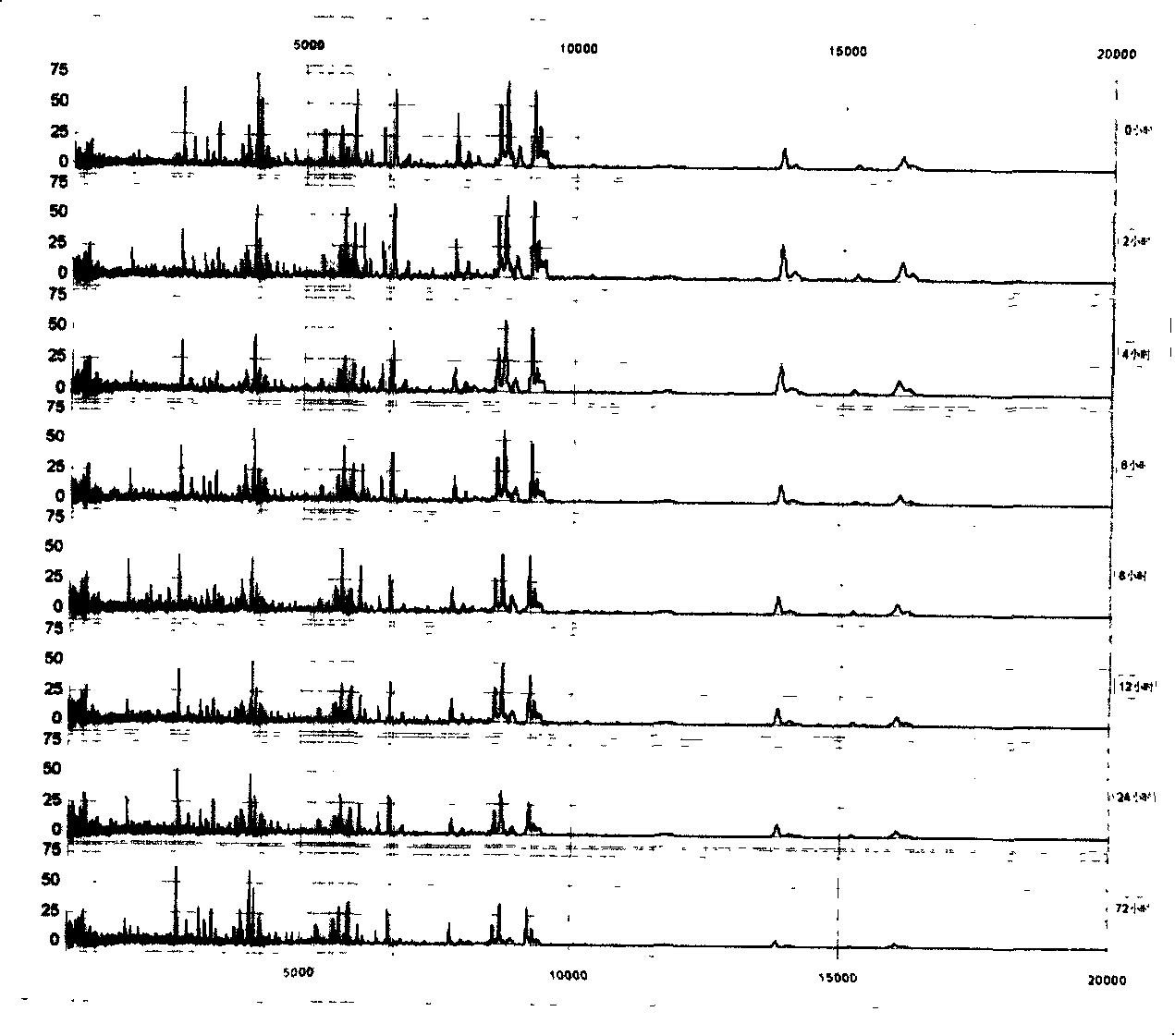
[0065] We put the serum (serum) at 4°C for different times: 0, 2, 4, 6, 8, 12, 24, and 72 hours, and found that within 2 hours, the proteome of the serum (serum) basically did not change, and the serum (serum) None of the 79 peaks defined in Slurry had a CV > 30%. , only 2 peaks (2.5%) had CV>20%. By 4 hours, there were 2 peaks (2.5%) CV>30% and 5 peaks (6.4%) CV>20%; after 12 hours, there were 5 peaks (6.4%) CV>30% and 10 peaks (12.7%) had CV > 20%. Time extended to 72 hours, there were 10 peaks (accounting for 12.7%) CV>30% and 19 peaks (accounting for 24.1%) CV>20% ( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com