Method for synthesizing chloro-aniline by chloronitrobenzene selective hydrogenation in alcohol-water system
A kind of technology of chlorinated nitrobenzene and chlorinated aniline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
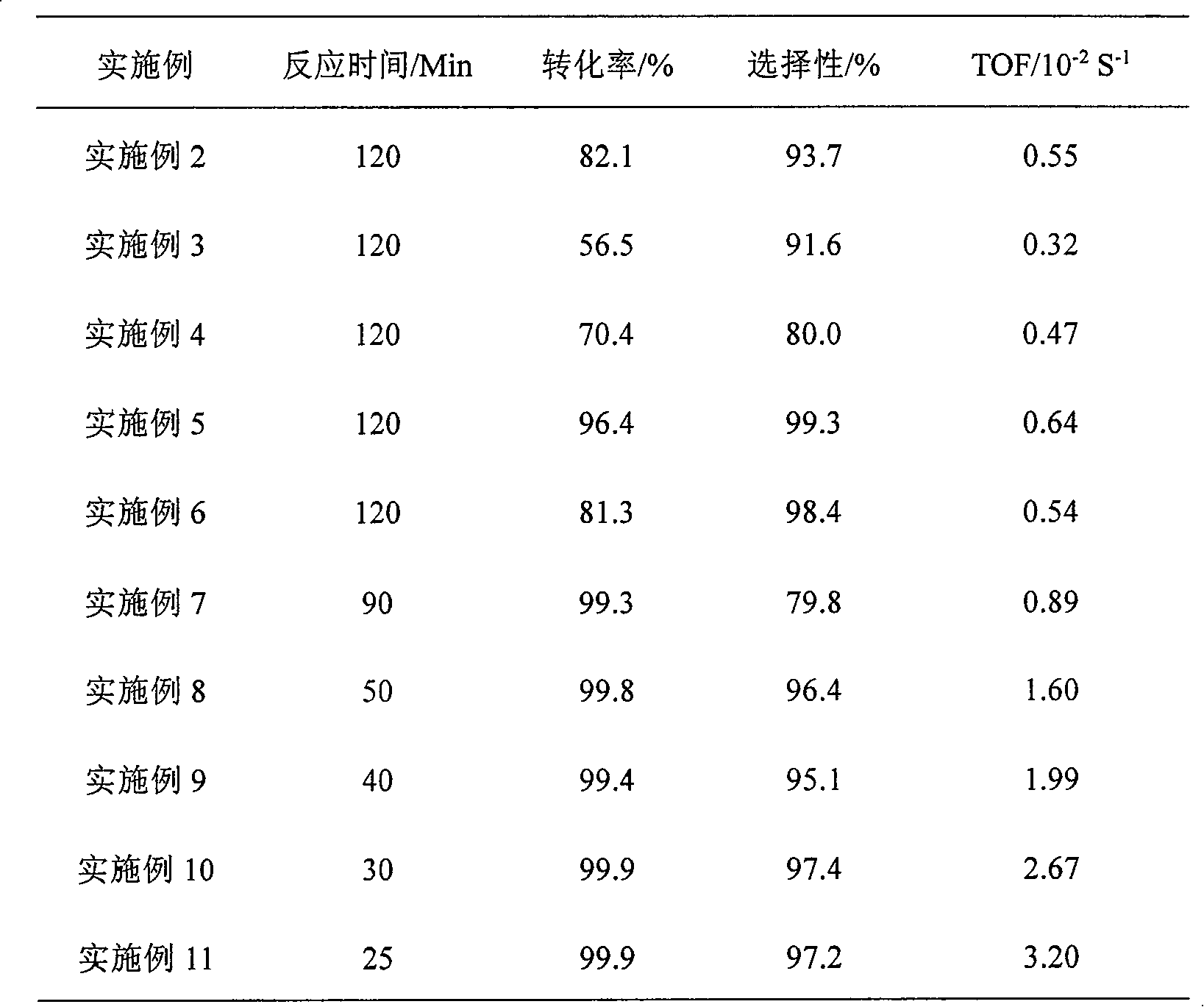
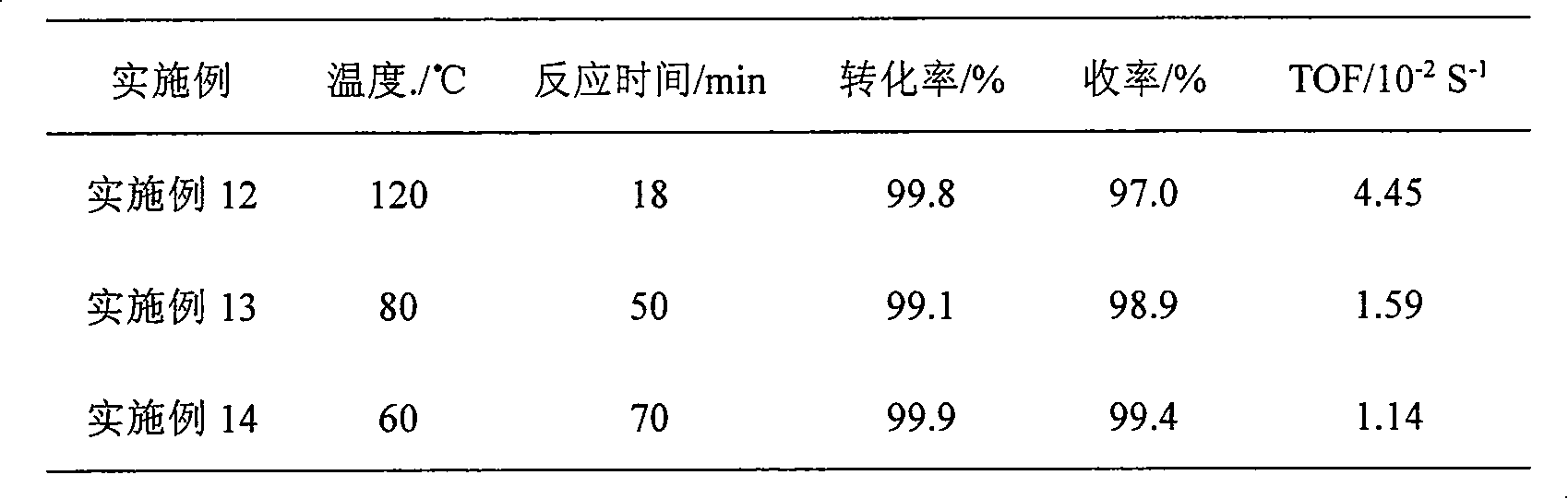
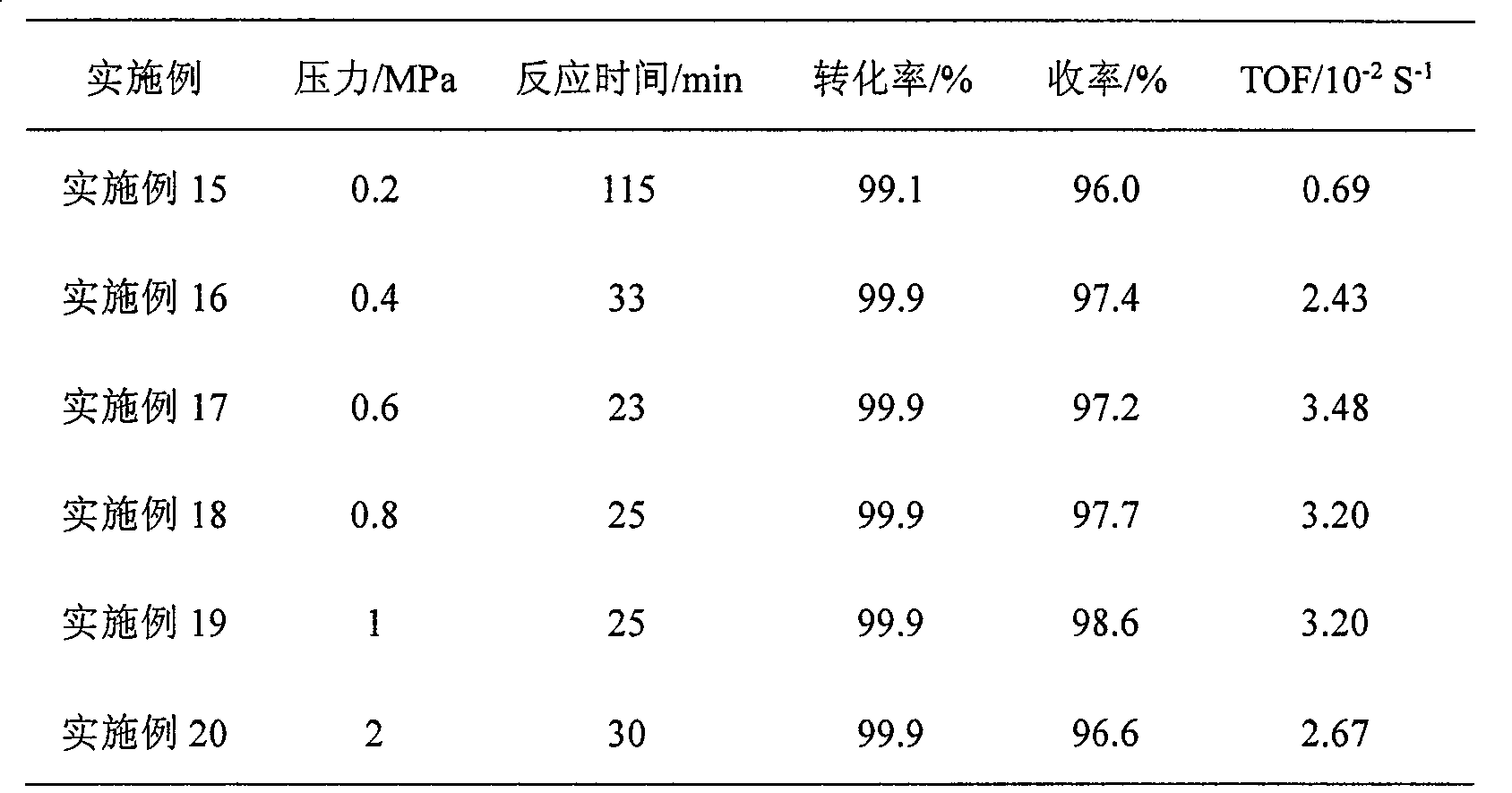
Embodiment 1
[0018] The reaction was carried out in a 500ml stainless steel autoclave, and 0.4g of Ru / SiO prepared by impregnation method 2 After the catalyst is reduced with potassium borohydride solution, it is used after washing and centrifuging with water and ethanol. Add 1.5g of p-chloronitrobenzene, 105ml of absolute ethanol and 45ml of water to the reactor, close the reactor, and replace it 3 times to get rid of air. It was heated to 100°C under stirring to start the reaction. The reaction was completed within 25min, and the content of each component of the reaction solution was analyzed by gas chromatography. The conversion rate was 99.9%, the yield was 98.6%, and the TOF was 3.20×10 -2 S -1 .
Embodiment 2
[0020] The reaction was carried out in a 500ml stainless steel autoclave, 0.4g Ru / SiO 2 After the catalyst is reduced with potassium borohydride solution, it is used after washing and centrifuging with water and ethanol. Add 1.5g of p-chloronitrobenzene and 150ml of methanol to the reaction kettle, close the reactor, and replace it 3 times to exclude air. Heated to 100°C to start the reaction. After reacting for 120 minutes, the content of each component of the reaction solution was analyzed by gas chromatography. The reaction results are shown in Table 1.
Embodiment 3
[0022] Except using 150ml butanol as solvent, other steps are the same as in Example 3, and the reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com