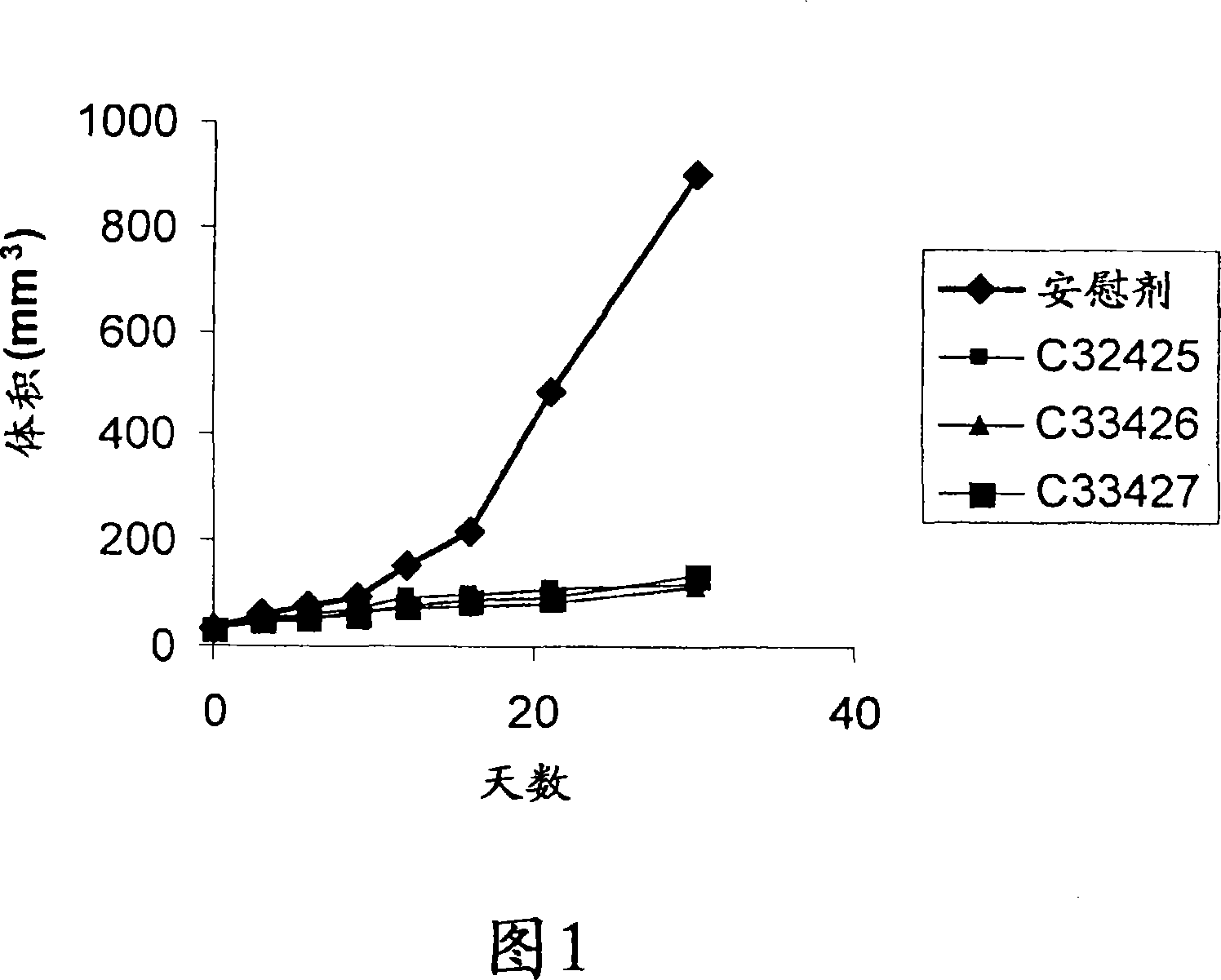
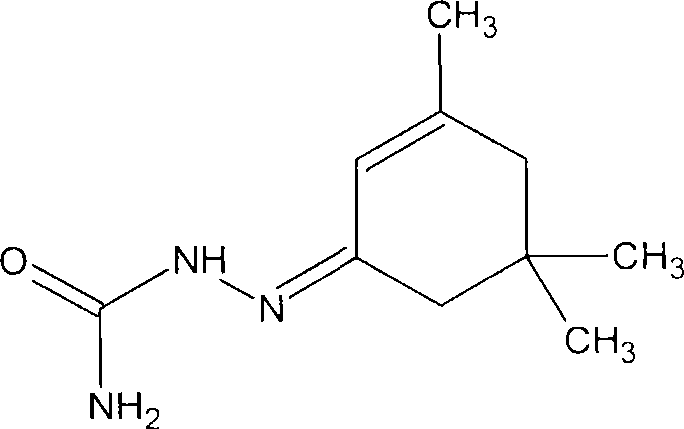
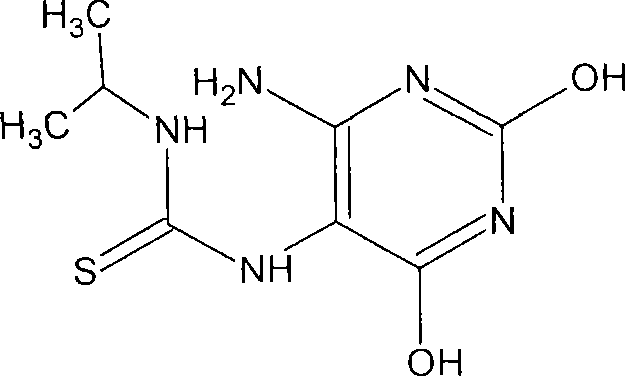
Antineoplastic compounds and pharmaceutical compositions thereof
一种化合物、底物的技术,应用在抗肿瘤药、药物组合、周期表第5/15族元素的化合物等方向,能够解决降低体内稳定性、不能渗透、不容易转运等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0194] Example 1: Compound selection by in silico molecular modeling
[0195] Several compounds were selected based on the higher values of the calculated binding energies of the receptor-ligand complexes (Table 1 ) by using a computational model developed from an extensive and substantial screen. This approximate energy value was estimated taking into account a comprehensive analysis of the conformation and several high-energy components using a computational program developed in our laboratory.
[0196] Table 1: Calculated interaction energies for receptor-ligand complexes
[0197] compound
Embodiment 2
[0198] Example 2: Effect of the Compounds on Phosphorylation of Typical CK2 Substrates
[0199]The assay involves performing an in vitro phosphorylation reaction using oncoprotein E7 derived from human papillomavirus type 16 (VHP-16), which acts as a glutathione S-transferase (GST) in Escherichia coli, as a substrate. Fusion protein expression. The resulting E7-GST was then purified by glutathione-agarose (Pharmacia) affinity chromatography. Before the enzymatic reaction, E7-GST was pre-incubated for 1 hour at 37°C, where different concentrations of compounds were used. The reaction was prepared by 50μl Tris: HCL 25mM pH 7.5 buffer, 1μCi 32 P-γATP, 100 μM ATP, 40 μl of resin containing E7-GST, 0.2M NaCl, 10 mM MgCl and 1 unit of CK2 enzyme (Promega) were carried out at 37°C for 40 minutes. After the reaction, the resin was washed 3 times with 0.5 ml of reaction buffer and finally the phosphorylation level of E7-GST was analyzed in 10% polyacrylamide gel electrophoresis (PAG...
Embodiment 3
[0203] Example 3: Effects of the Compounds on Phosphorylation of the CK2 Consensus Site
[0204] This assay involves performing an in vitro phosphorylation reaction using as a substrate the sequence RRREEETEEE, which is generally recognized as the optimal consensus phosphorylation domain for CK2 substrates.
[0205] The substrate peptides were pre-incubated for 1 hour at 37°C prior to the enzymatic reaction, using different concentrations of compounds. The reaction was prepared by 50μl Tris: HCL 25mM pH7.5 buffer, 1μCi 32 P-γATP, 100 μM ATP, 40 μl of E7-GST-containing resin, 0.2 M NaCl, 10 mM MgCl and 1 unit of CK2 enzyme (Promega) were incubated at 37°C for 10 minutes. After the reaction, 5 μl of the reaction mixture was spread on Whatmann PE-81 filter paper and washed with 10 mM H 3 PO 4 After washing four times, the filter-associated radioactivity was finally measured and the cpm values of each sample were directly related to CK2 enzymatic activity.
[0206] IC50 valu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com