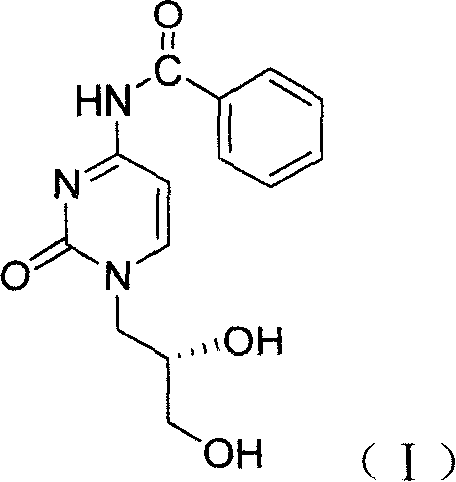
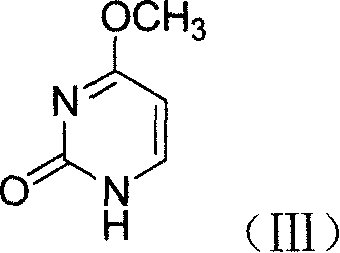
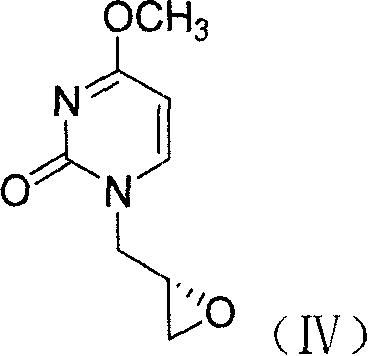
Method for preparing cidofovir key intermediate
A compound, -N4- technology, applied in organic chemistry and other directions, can solve the problems of many side reactions, high cost, application limitations, etc., and achieve the effect of reducing reaction cost, improving yield and reducing side reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Preparation of (S)-N-(2,3-epoxypropyl)-4-methoxy-2-pyrimidinone (IV)
[0033] N 2 Under protection, 4-methoxy-2-pyrimidinone (12.6g, 0.1mol) was dissolved in dried DMF (1L), heated to 100°C, 80% NaH (9g, 0.3mol) was added, stirred 0.5h, add (R)-epichlorohydrin (9.25g, 0.1mol), react at 110°C for 2h, cool to room temperature, pour the reaction solution into a large amount of ice water, extract with ethyl acetate (300ml×3), combine acetic acid Ethyl ester was concentrated, and the oil was poured into petroleum ether (500ml), stirred, and filtered to obtain 11g of a yellow solid, with a yield of 61%.
[0034] 2. Preparation of (S)-N-(2,3-epoxypropyl)-cytosine (V)
[0035] Compound (IV) (11 g, 0.06 mol) was added to 30% methylamino alcohol solution (200 ml), and reacted at 120° C. for 8 h. After the reaction was completed, it was concentrated under reduced pressure and recrystallized from ethanol to obtain 8.03 g of a dark yellow solid. Yield 80%.
[0036] 3. Prepara...
Embodiment 2
[0041] 1. Preparation of (S)-N-(2,3-epoxypropyl)-4-methoxy-2-pyrimidinone (IV)
[0042] N 2 Under protection, 4-methoxy-2-pyrimidinone (12.6g, 0.1mol) was dissolved in dried THF (1L), heated to 100°C, 80% NaH (9g, 0.3mol) was added, stirred 0.5h, add (R)-epibromohydrin (9.25g, 0.1mol), react at 110°C for 4h, cool to room temperature, pour the reaction solution into a large amount of ice water, extract with ethyl acetate (300ml×3), combine acetic acid Ethyl ester was concentrated, and the oil was poured into petroleum ether (500ml), stirred, and filtered to obtain 11g of a yellow solid, with a yield of 61%.
[0043] 2. Preparation of (S)-N-(2,3-epoxypropyl)-cytosine (V)
[0044] Compound (IV) (11 g, 0.06 mol) was added to 30% methylamino alcohol solution (200 ml), and reacted at 120° C. for 8 h. After the reaction was completed, it was concentrated under reduced pressure and recrystallized from methanol to obtain 8.03 g of a dark yellow solid. Yield 80%.
[0045] 3. Prepara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com