Immunosuppressant-anti-CD25 mosaic monoclonal antibody
A monoclonal antibody, CDR2 technology, applied in the fields of genetic engineering and immunology, can solve problems such as rejection, loss of target antigen affinity and stability, and expensive treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
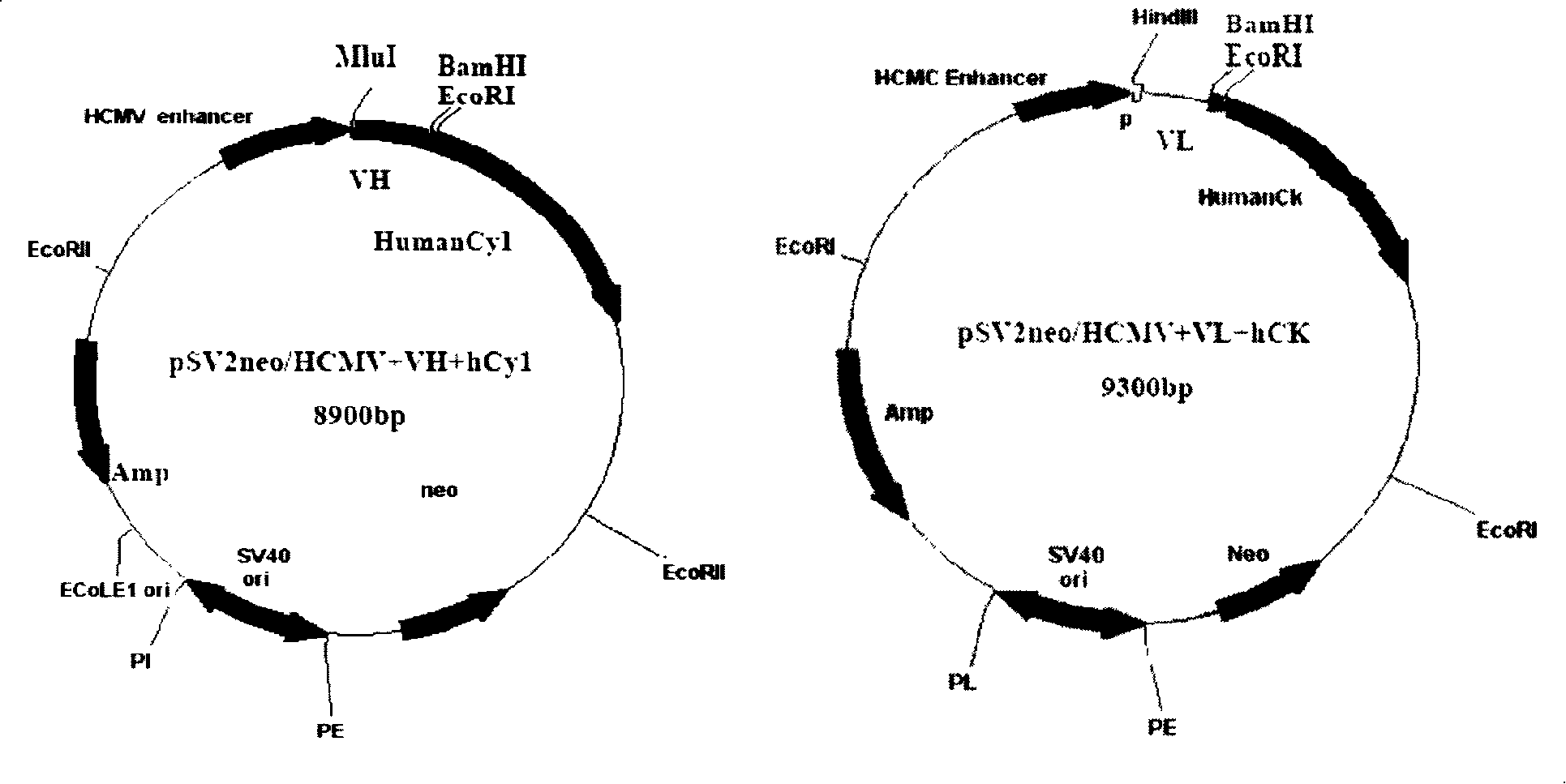
[0083] Expression vector construction and transformation
[0084] 1. Acquisition of heavy chain variable region VH and light chain variable region VL of anti-CD25 monoclonal antibody
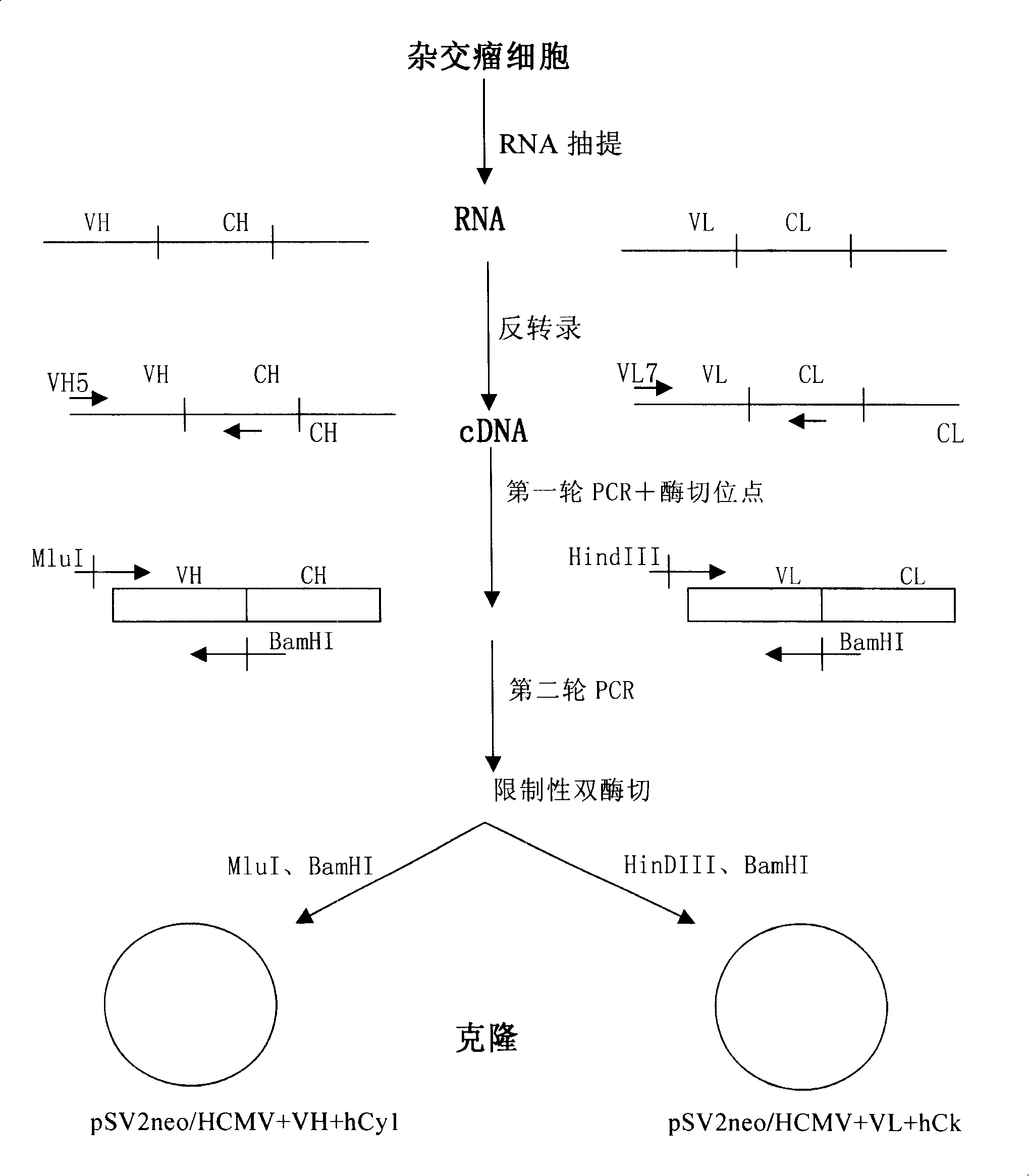
[0085] The steps for obtaining the heavy chain variable region VH and the light chain variable region VL of an anti-CD25 monoclonal antibody are as follows: figure 1 shown.
[0086] Human lymphocytes activated by phytohemagglutinin (PHA) were used to immunize LOU / C rats (purchased from the Experimental Animal Center of Chinese Academy of Medical Sciences), and then the splenocytes and non-secreting bone marrow cell line IR983F were used (see Bazin H (1982) Production of rat monoclonal antibodies with the LOU ratnon-secreting IR983F myeloma cell line. Protein Biol Fluids 29: 615-618) were fused to obtain hybridoma cells with anti-CD25 monoclonal antibody.
[0087] Total RNA (Gibco BRL. Gaithersburg. USA) was isolated from anti-CD25 monoclonal antibody-producing hybridomas using Trizol reagent a...
Embodiment 2
[0129] Selection of the best medium
[0130] One serum-free cell line recovered from the seed cell bank was inoculated into a T25 square bottle for cultivation. After 48 hours, the cells were inoculated into three T25 square bottles at a ratio of 1:3, and then the cells could be inoculated at a ratio of 1:3. Carry out expansion culture.
[0131] Cells were inoculated into different media at the same density, and the number and expression of cells were investigated after 48 hours. The media tested are listed in Table 2.
[0132] Table 2
[0133]
[0134] In Table 2, BD CHO medium was purchased from BD Company of the United States, Excell 620 medium was purchased from JRH Company, and Pramatone and DOMA were purchased from Life Technologies Company.
[0135] The experimental data showed that the cell growth rate and the expression level of the product reached the highest in the B+E+P medium, and the expression level of the antibody exceeded 100 mg / L. Therefore, B+E+P medi...
Embodiment 3
[0137] Study on High Density Serum-free Suspension Culture of Engineering Cells
[0138] The present inventor investigated the adaptation process of engineered cells producing anti-CD25 chimeric monoclonal antibody in BD CHO, BD CHO+excel 620+Pramatone, Excell 620+DOMA+Pramatone and Excell 620 medium, and the results are shown in Table 3 .
[0139] table 3
[0140] culture medium
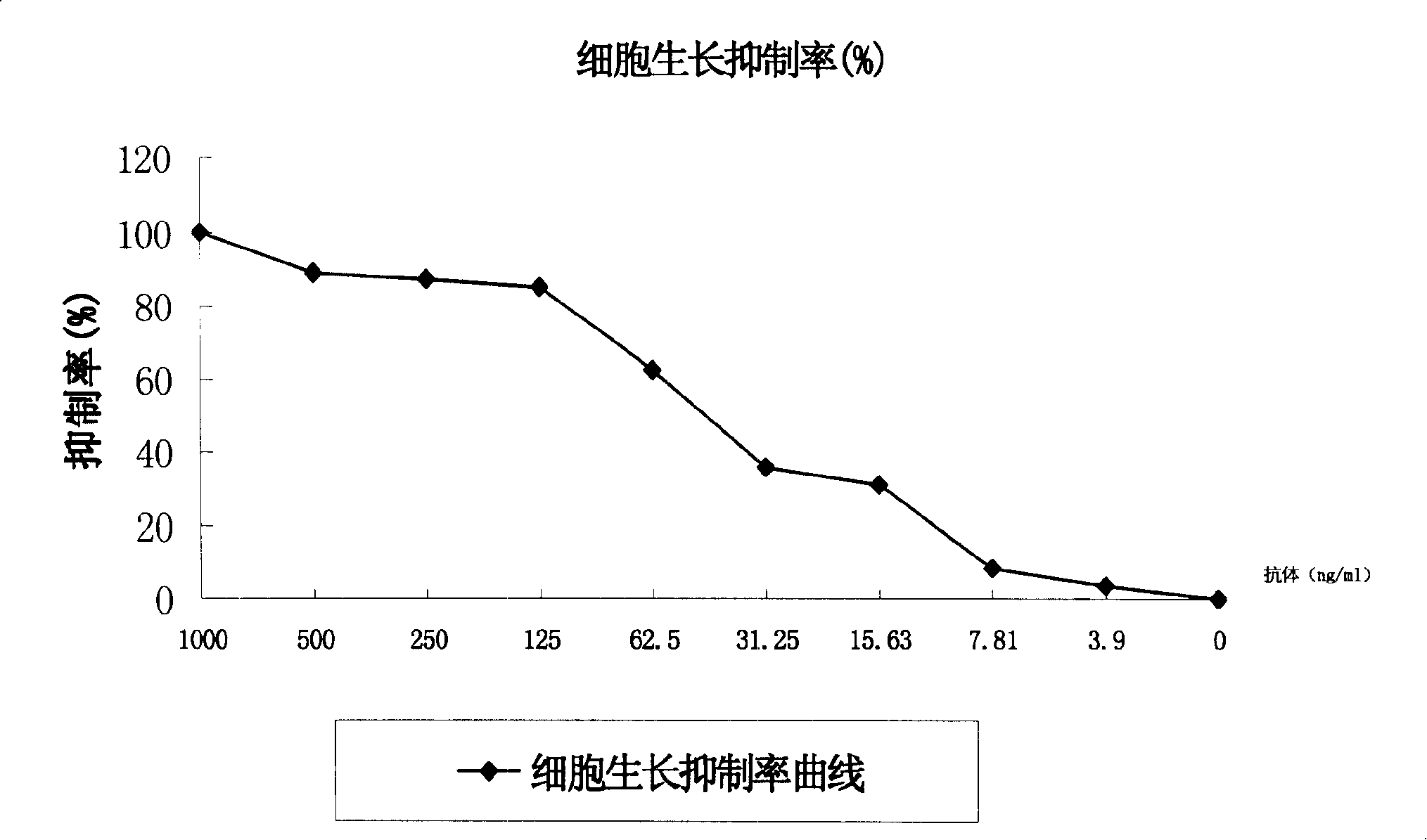
[0141] To sum up, it is preferable to use BD CHO+Excel 620+Pramatone medium as the serum-free medium for cell expansion, expand and culture the cells in a 250ml spinner, obtain a certain amount of culture supernatant, and provide purification for the purification process Research, and obtain a certain amount of pure product to provide quality control for structural confirmation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com