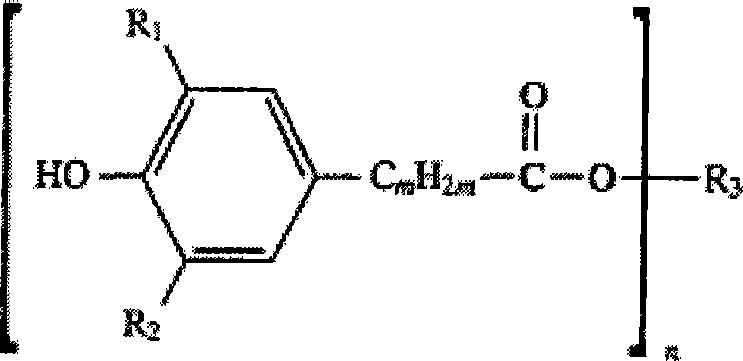
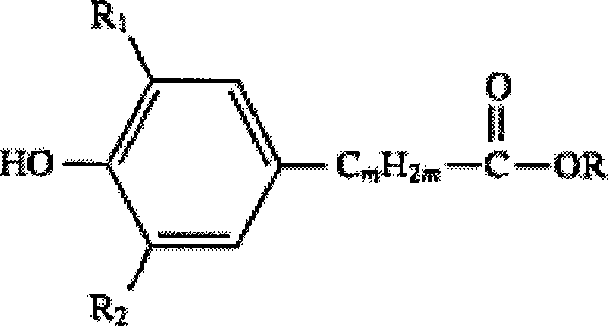
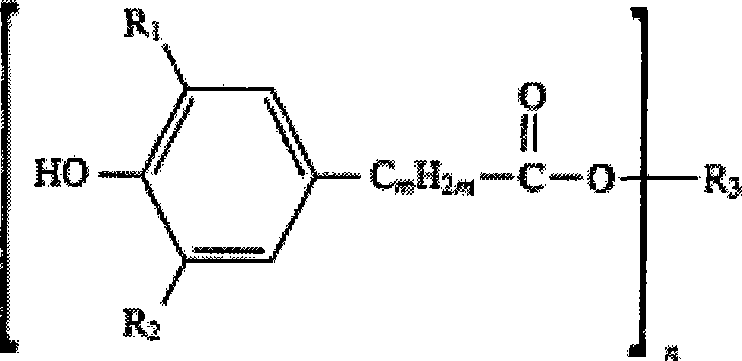
Method for synthesizing hindered phenol antioxidants
A synthesis method and technology of hindered phenols are applied in the synthesis field of oxidants, which can solve the problems of affecting the color and luster of antioxidants, low activity and the like, and achieve the effects of high production efficiency, high activity and excellent product performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthetic method of β-(3,5-di-tert-butyl-4-hydroxyphenyl) octadecyl propionate (antioxidant 1076)
[0038] 220.0 grams of 3,5-di-tert-butyl-4-hydroxyphenylpropionic acid methyl ester (abbreviated as 35 methyl ester) and 180.0 grams of carbon octadecyl alcohol are joined in a 1000ml four-necked flask with a stirring bar and a thermometer, and the temperature is raised to Melt the material at 70°C, then vacuumize, keep the vacuum at 20mmHg for 0.5 hours, pass N 2 , remove the vacuum, then under the protection of nitrogen, add 0.73 grams of zinc isooctanoate, turn off the nitrogen, vacuumize, and start to heat up to 185 ° C at the same time, at the initial stage of the reaction, because there is more methanol produced, the vacuum degree is 10 ~ 20mmHg, reaction 2 After one hour, the vacuum was controlled at 3 to 5 mmHg, and the reaction was continued for 4 hours.
[0039] After the reaction was completed, the temperature was raised to 195° C., and 27.1 g of unreacted 35 m...
Embodiment 2
[0042] Synthetic method of β-(3,5-di-tert-butyl-4-hydroxyphenyl) octadecyl propionate (antioxidant 1076)
[0043] 200.0 grams of 3,5-di-tert-butyl-4-hydroxyphenylpropionic acid methyl ester (abbreviated as 35 methyl ester) and 220.0 grams of carbon octadecyl alcohol are added in a 1000ml four-necked flask with a stirring bar and a thermometer, and the temperature is raised to Melt the material at 70°C, then vacuumize, keep the vacuum at 20mmHg for 0.5 hours, pass N 2 , remove the vacuum, then under the protection of nitrogen, add 0.73 grams of zinc isooctanoate, turn off the nitrogen, vacuumize, and start to heat up to 185 ° C at the same time, at the initial stage of the reaction, because there is more methanol produced, the vacuum degree is 10 ~ 20mmHg, reaction 2 After one hour, the vacuum was controlled at 3 to 5 mmHg, and the reaction was continued for 4 hours.
[0044] After the reaction was completed, the temperature was raised to 195° C., and 35.3 grams of unreacted i...
Embodiment 3
[0047] Synthetic method of bis[β-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate] hexanediol ester (antioxidant 259)
[0048] Add 358.2 grams of 3,5-di-tert-butyl-4-hydroxyphenylpropionic acid methyl ester (referred to as 35 methyl ester) and 60.0 grams of hexanediol into a 1000ml four-necked flask with a stirring bar and a thermometer, and heat up to 90°C , then, vacuumize, keep the vacuum at 10mmHg for 0.5 hours, pass N2, remove the vacuum, then add 1.10 g of zinc isooctanoate under nitrogen protection, turn off the nitrogen, vacuumize, and start to heat up to 165 ° C at the same time, the initial stage of the reaction , because more methanol is produced, the vacuum degree is 10-20 mmHg, after 2 hours of reaction, the vacuum degree is controlled at 3-5 mmHg, and the reaction is continued for 3 hours.
[0049] After the reaction was completed, the temperature was raised to 195° C., and 58.2 grams of unreacted 35 methyl esters were distilled off. The temperature was lowered to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com