Synthesis method of clofarabine, midbody thereof and preparation method of midbody
A synthetic method, the technology of clofarabine, which is applied in the direction of preparation of sugar derivatives, chemical instruments and methods, esterified saccharides, etc., can solve the problems that are not suitable for industrial production, and achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
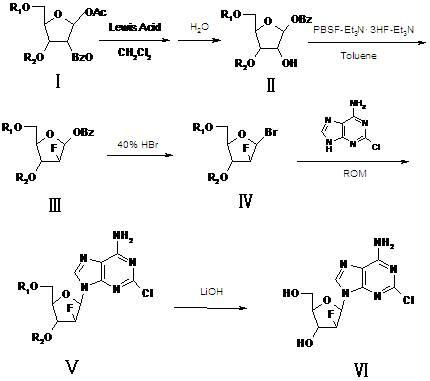
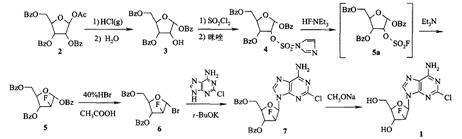
[0026] A kind of synthetic method of clofarabine comprises the following steps:
[0027] 1) Compound Ⅰ undergoes a rearrangement reaction with a strong Lewis acid in dichloromethane, and then undergoes hydrolysis to generate compound Ⅱ;
[0028] 2) Compound II undergoes a fluorination reaction with a fluorinating agent and triethylamine in toluene to generate Compound III;
[0029] 3) Compound III reacts with hydrogen bromide to generate compound IV;
[0030] 4) Condensation reaction of compound IV with 2-chloroadenine, ROM to generate compound V;
[0031] 5) Compound V is reacted with lithium hydroxide to prepare the clofarabine;
[0032] R1 and R2 of compound I in the step 1) are benzoyl or acetyl, and the molar ratio of the fluorinating agent and triethylamine in the step 2) is: 1:1~2, the best is 1:2, R in the ROM in step 4) is a C1~C4 alkyl group, M is K, Na, Li, preferably lithium tert-butoxide.
[0033] The strong Lewis acid used in the step 1) includes titanium...
Embodiment 1
[0039] Preparation of compound Ⅱ
[0040] In an ice-water bath, put 30 g of compound I into a 1000 mL reaction flask, add 250 mL of dichloromethane, and stir magnetically to dissolve it. 11.7 g of titanium tetrachloride was slowly added dropwise within 40 minutes at 0 °C, and continued to stir for 20 minutes. Heat up to 15°C, add 100 mL of distilled water, continue stirring at this temperature for 6 hours, separate the water phase, extract the water phase with 20 mL of dichloromethane, combine the organic phases, adjust the pH of the solution to 7 with saturated sodium bicarbonate, The organic phase was dried over anhydrous sodium sulfate, filtered, evaporated to remove the solvent under reduced pressure, dried in vacuo, added 400 mL of methanol and heated to reflux, and completely dissolved the solid, cooled slowly to room temperature, then placed in a refrigerator for crystallization, suction filtered, and vacuum-dried. Dry to obtain 18.1 g of white solid, total yield 63.7%...
Embodiment 2
[0042] Preparation of compound Ⅱ
[0043]In an ice-water bath, put 45 g of compound I into a 1000 mL reaction flask, add 400 mL of dichloromethane, and stir magnetically to dissolve it. 17.5 g of titanium tetrachloride was slowly added dropwise within 80 minutes at -20 °C, and continued to stir for 40 minutes. Heat up to 0°C, add 150 mL of distilled water, continue to stir at this temperature for 8 hours, separate the water phase, extract the water phase with 30 mL of dichloromethane, combine the organic phases, adjust the pH of the solution to 7 with saturated sodium bicarbonate, Dry the organic phase with anhydrous sodium sulfate, filter, distill off the solvent under reduced pressure, dry in vacuum, add 600 mL of methanol and heat to reflux, and completely dissolve the solid, cool slowly to room temperature, then put it in the refrigerator to crystallize, filter with suction, vacuum Dry to obtain 25.4 g of white solid, total yield 58.9%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com