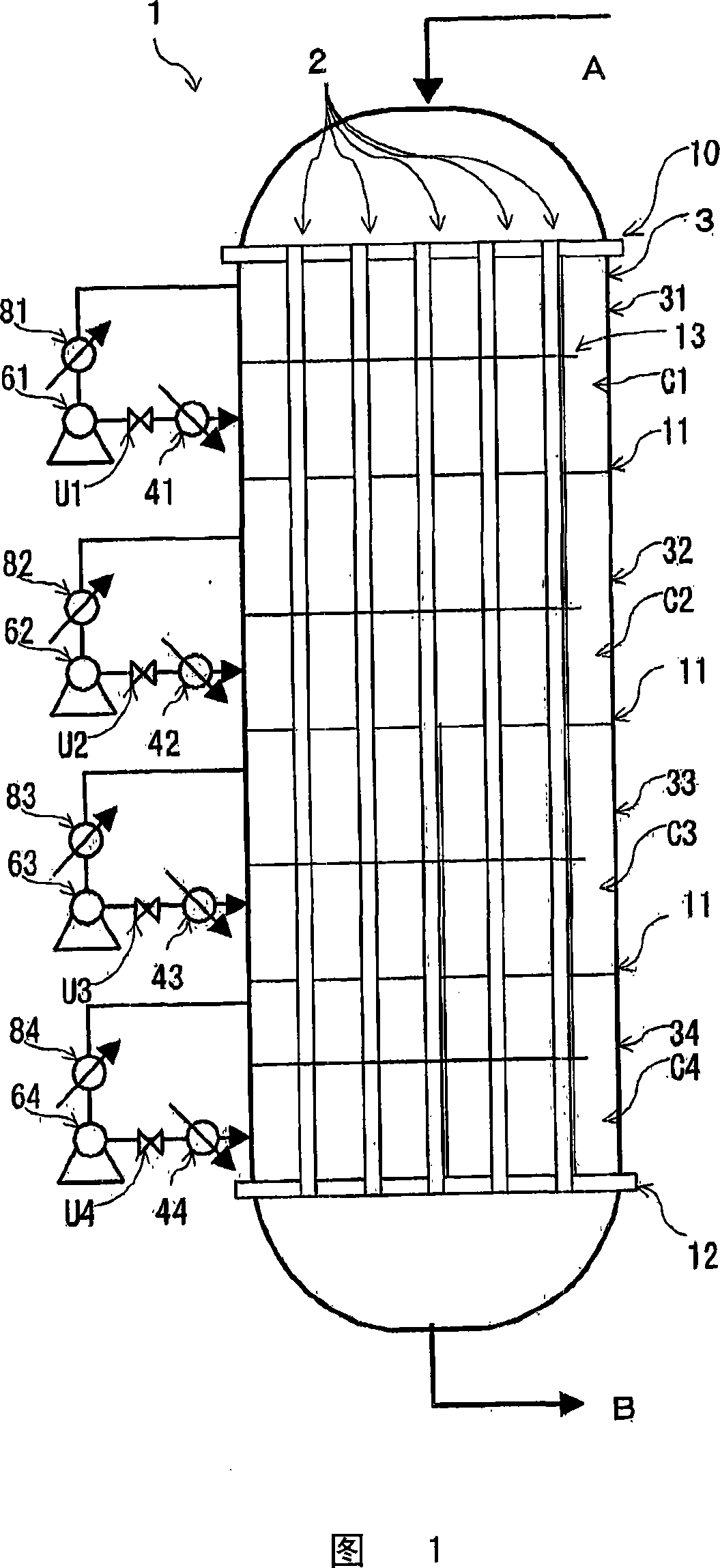
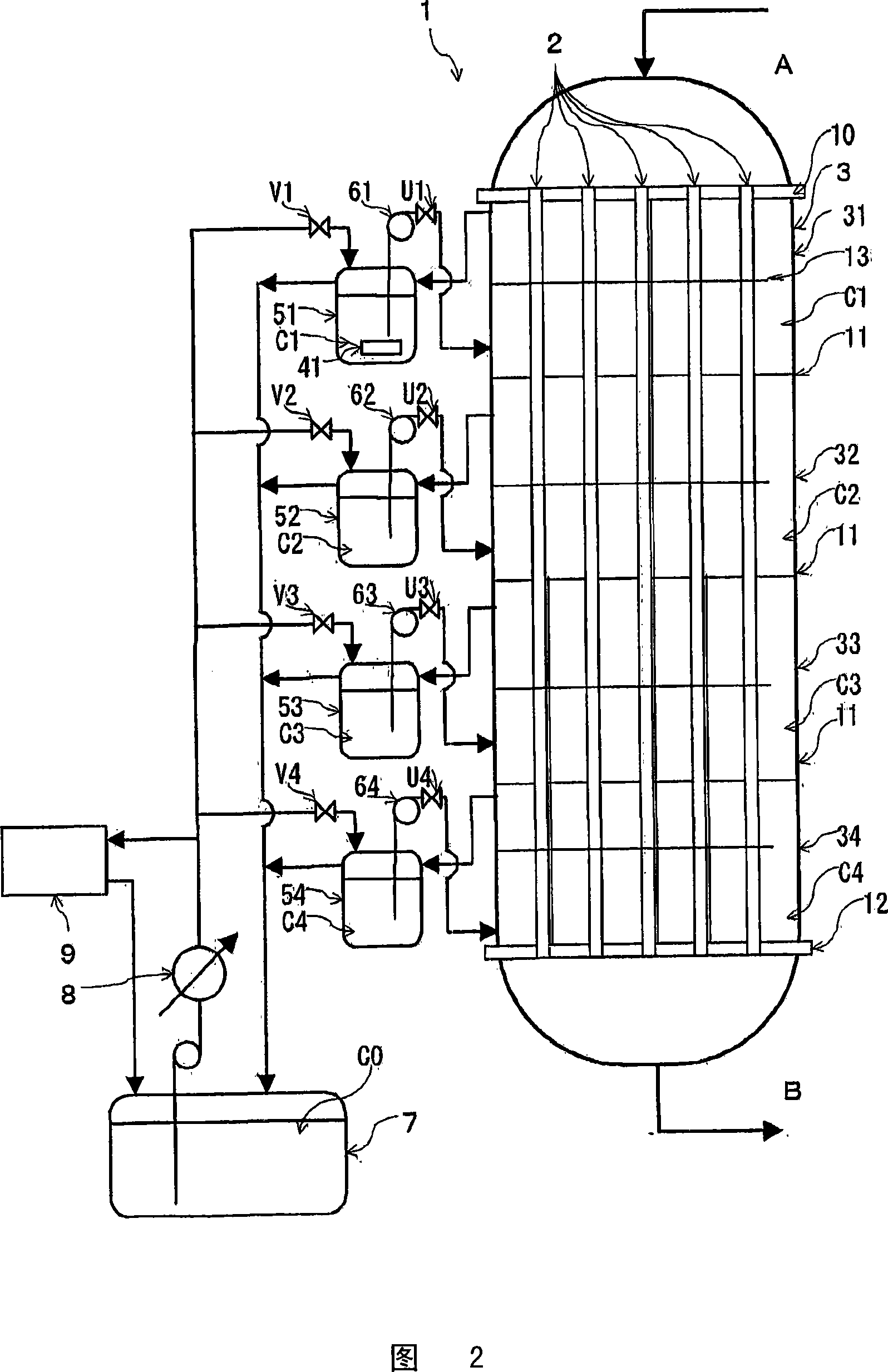
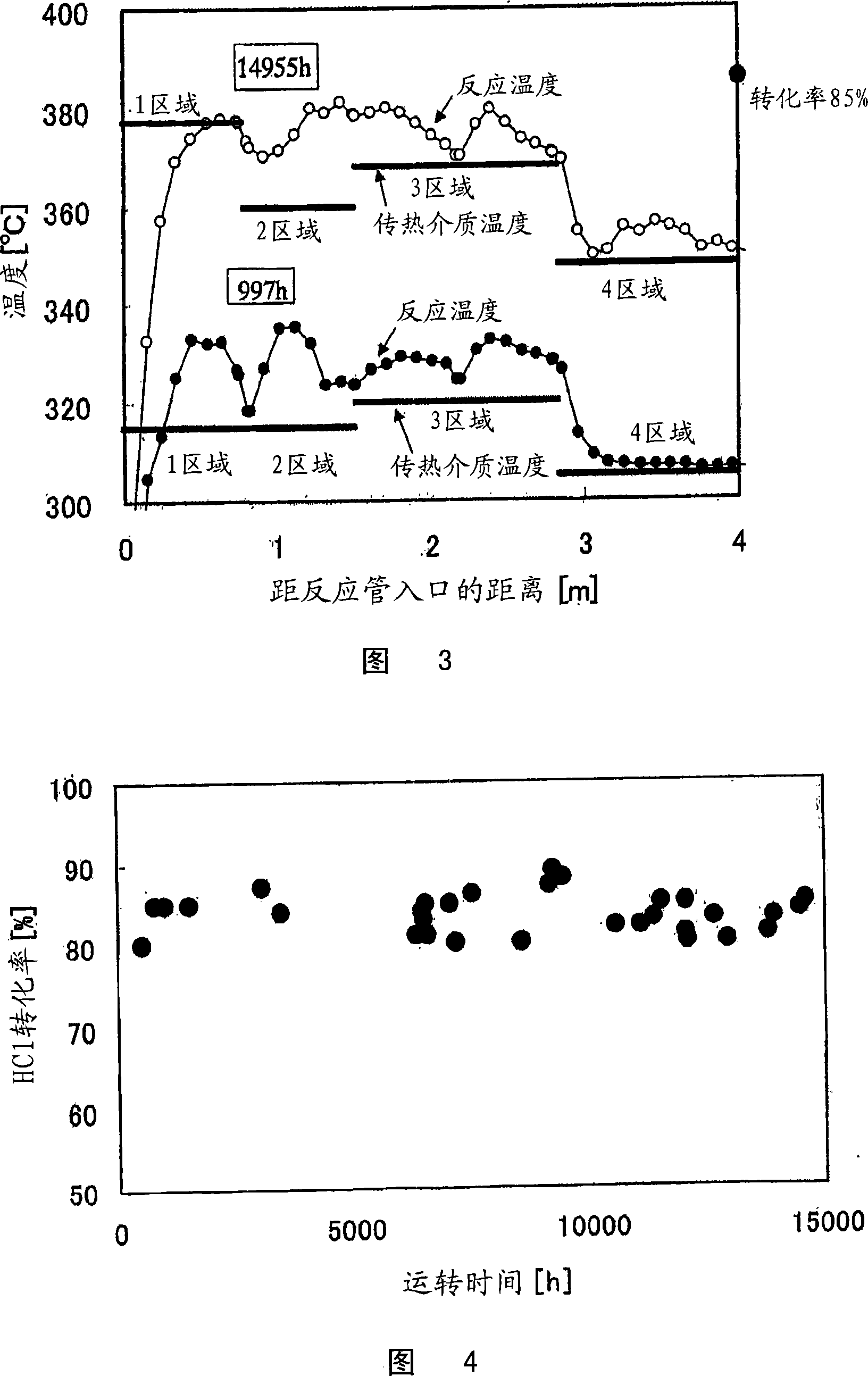
Reactor for chlorine production and process for producing chlorine
A reactor and reaction tube technology, applied in chemical instruments and methods, preparation with chloride, chlorine/hydrogen chloride, etc., can solve the problem of insufficient catalyst life, and achieve the effect of stably maintaining catalytic activity and effectively utilizing catalyst.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0102] Hereinafter, the present invention will be described in more detail using examples, but the present invention is not limited by the examples.
[0103] [1] Preparation of catalyst
[0104] (1) Catalyst 1:
[0105] Titanium oxide and α-alumina were mixed in a mass ratio of 34:66 (titanium oxide:alumina), and then pure water was added and kneaded. The mixture was extruded into a cylindrical shape having a diameter of 1.5 mmφ, dried, and then crushed into a length of about 2 to 4 mm. The obtained molded product was fired at 600° C. for 3 hours in the air to obtain a support composed of a mixture of titanium oxide and α-alumina.
[0106] The carrier was impregnated with an aqueous solution of ruthenium chloride, dried, and then fired in air at 250° C. for 2 hours to obtain a supported oxide in which ruthenium oxide was supported on the carrier at a loading rate of 1% by mass. ruthenium.
[0107] (2) Catalyst 2
[0108] Titanium oxide and α-alumina were mixed in a mass r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com