Disubstituted allyl benzene derivatives as well as preparation and uses thereof
A technology of substituents and compounds, applied in the field of disubstituted phenylpropene derivatives and their preparation and use, can solve the problems of unsatisfactory, limited universal applicability of drugs, malignant killing of normal cells and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
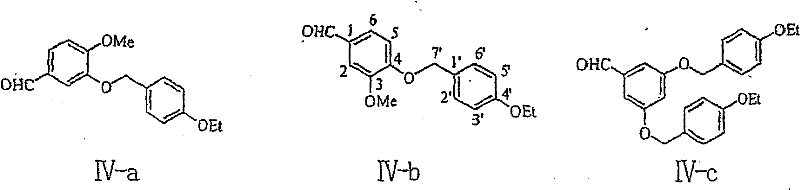
[0065] Example 1: Preparation of compound IV-d (3,4-bis(4-ethoxybenzyloxy)-benzaldehyde)
[0066]
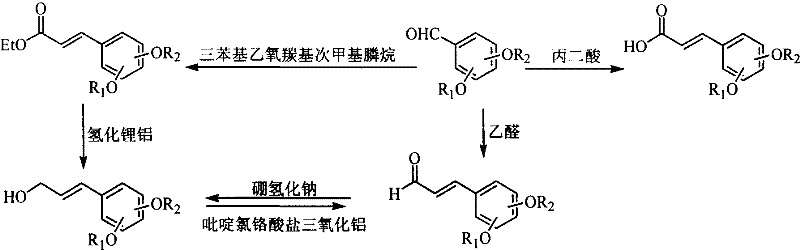
[0067] This example relates to a general synthesis method of a class of 3,4-disubstituted phenylpropene derivative key intermediates 3,4-disubstituted benzaldehyde series compounds with cytotoxic activity shown in formula (IV). It specifically relates to the synthesis of compound 3,4-bis(4-ethoxybenzyloxy)-benzaldehyde. In a 25 mL three-necked flask, a solution of p-ethoxybenzyl bromide (400 mg, 1.86 mmol) in N,N-dimethylformamide (4.0 mL) was added to potassium carbonate (200 mg, 1.45 mmol ) and 3,4-dihydroxybenzaldehyde (100 mg, 0.72 mmol) in N,N-dimethylformamide (4.0 ml) mixture, refluxed for 3 hours. Cooled to room temperature, filtered, and the filtrate was concentrated and passed through the column to obtain 180 mg of white solid with a yield of 61.2%.
[0068] Compound IV-d: white solid, melting point: 98-100°C, R f (n-hexane / ethyl acetate: 1 / 1) 0.73; 1 H NMR (40...
Embodiment 2
[0069] Example 2: Preparation of compound IV-c (3,5-bis(4-ethoxybenzyloxy)-benzaldehyde)
[0070]
[0071] This example relates to a general synthesis method of a class of 3,5-disubstituted phenylpropene derivative key intermediate 3,5-disubstituted benzaldehyde series compounds having cytotoxic activity shown in formula (IV). It specifically relates to the synthesis of compound 3,5-bis(4-ethoxybenzyloxy)-benzaldehyde. Add 3,5-bis(4-ethoxybenzyloxy)-benzohydrazide (1.05 g, 2.41 mmol), tetrabutylammonium bromide (0.78 g, 2.41 mmol), ammonia (25%, 2.4 milliliters) and benzene (20 milliliters), were added dropwise to potassium ferricyanide solution, and after stirring for 1 hour, the product was extracted with an organic solvent, and the filtrate was concentrated and passed through a column to obtain 250 milligrams of a white solid , the yield was 22.1%.
[0072] Compound IV-c: white solid, melting point: 97-99°C, R f (n-hexane / ethyl acetate: 3 / 1): 0.40; 1H NMR (400MHz, C...
Embodiment 3
[0073] Example 3: Preparation of compound IV-e (2,4-bis(4-ethoxybenzyloxy)-benzaldehyde)
[0074]
[0075] This example relates to a general synthesis method of a class of 2,4-disubstituted phenylpropene derivative key intermediate 2,4-disubstituted benzaldehyde series compounds having cytotoxic activity shown in formula (IV). It specifically relates to the synthesis of compound 2,4-bis(4-ethoxybenzyloxy)-benzaldehyde. In a 25 mL three-necked flask, a solution of p-ethoxybenzyl bromide (400 mg, 1.86 mmol) in N,N-dimethylformamide (4.0 mL) was added to potassium carbonate (200 mg, 1.45 mmol ) and 2,4-dihydroxybenzaldehyde (100 mg, 0.72 mmol) in N,N-dimethylformamide (4.0 ml) mixture, refluxed for 3 hours. Cool to room temperature, filter, concentrate the filtrate, and recrystallize from chloroform and methanol to obtain 150 mg of IV-e colorless crystals, with a yield of 51.0%.
[0076] Compound IV-e: colorless crystals, melting point: 116-118°C, R f (n-hexane / ethyl acet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com