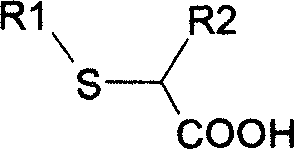
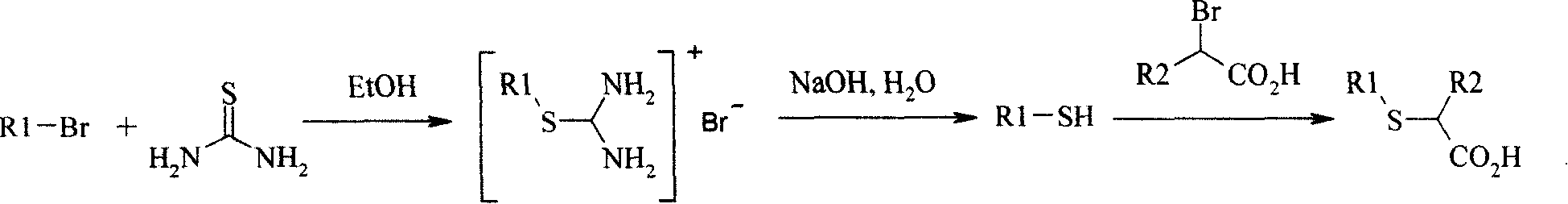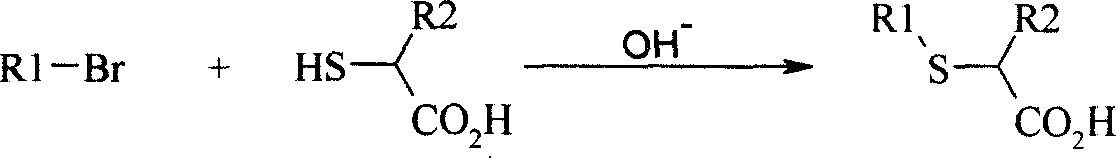New method for preparing neta-thia-alpha-alkyl fatty acid
A technology of alkyl fatty acid and a new method, applied in the direction of sulfide preparation, organic chemistry, etc., can solve the problems of high production cost, many steps in the reaction process, and easy oxidation, etc., and achieve simple and stable production process, easy to obtain materials on site, The effect of little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1.3-thia-2-methyltridecanoic acid
[0035] At room temperature, add 15.94g of sodium hydroxide (excessive) into 200ml of methanol, stir to dissolve it, add dropwise 18.3ml (21.88g, 0.206mol) of 2-mercaptopropionic acid, then dropwise add 22.1g (0.1mol) of 1- Bromodecane, react for 1.5-2 hours, a large amount of white solid precipitates, continue to stir until the reaction is complete, then add water and hydrochloric acid to the system to acidify, stir and mix well, filter the separated precipitate with suction, wash with water, and recrystallize from ethanol , 17.2g of crystals were obtained, the yield was 75%, mp.38.1-38.3°C, and the purity by GC analysis was 97.0%.
[0036] The obtained product is analyzed as follows:
[0037] White scaly crystals;
[0038] mp.38.1-38.3°C (recrystallized from EtOH);
[0039] IR(KBr)ν max : 2960, 2920, 2870, 1700, 1470, 1250cm -1 .
[0040] 1 H NMR (300MHz, CDCl 3 ): δ10.13 (bs, OH), 3.43 (q, 1H, J...
Embodiment 2
[0043] The preparation of embodiment 2.3-thia-2-methylpentadecanoic acid
[0044] At room temperature, add 17.4g of sodium hydroxide (excessive) into 200ml of methanol, stir to dissolve it, add dropwise 18.3ml (21.88g, 0.206mol) of 2-mercaptopropionic acid, and then add 24.9g (0.1mol) of decabromide Dioxane, reacted for 1.5-2 hours, a large amount of white solid precipitated, continued to stir until the reaction was complete, then added water and hydrochloric acid to the system, stirred, suction filtered the precipitate, washed with water, recrystallized from ethanol, filtered to obtain 20.6 g crystallization, yield 75.4%, mp. 51-52°C, GC analysis purity 97.0%.
[0045] The obtained product is analyzed as follows:
[0046] White scaly crystals;
[0047] mp.51-52°C (recrystallized from EtOH);
[0048] IR(KBr)ν max : 2960, 2920, 2870, 1700, 1470, 1250cm -1 ;
[0049] 1 H NMR (300MHz, CDCl 3 ): δ10.13 (bs, OH), 3.43 (q, 1H, J=6.6Hz, -SC H COOH), 2.59-2.69 (m, 2H, -C H 2 ...
Embodiment 3
[0052] The preparation of embodiment 3.3-thia-2-methylheptadecanoic acid
[0053] At room temperature, add 17.4g of sodium hydroxide (excess) into 200ml of methanol, stir to dissolve it, add dropwise 18.3ml (21.88g, 0.206mol) of 2-mercaptopropionic acid, and then add 25ml (27.7g, 0.1mol) of bromine Tetradecane, reacted for 1.5-2 hours, a large amount of white solids were precipitated, continued to stir until the reaction was complete, then added water and hydrochloric acid to the system, stirred, suction filtered the separated precipitates, washed with water, and recrystallized from ethanol to obtain 24.1 g crystallization, yield 79.8%, mp. 51.8-52.2°C, GC analysis purity 97.5%.
[0054] The obtained product is analyzed as follows:
[0055] White scaly crystals;
[0056] mp.51.8-52.2°C (recrystallized from EtOH);
[0057] IR(KBr)ν max : 2960, 2920, 2870, 1700, 1470, 1250cm -1 ;
[0058] 1 H NMR (300MHz, CDCl 3 ): δ10.13 (bs, OH), 3.41 (q, 1H, J=7.2Hz, -SC H COOH), 2.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com