Method for preparing hydroperoxidation p-menthane by catalytic air oxidation p-menthane
A technology of hydrogen peroxide and air oxidation, which is applied to the preparation of peroxygen compounds, chemical instruments and methods, and the preparation of organic compounds, etc. It can solve the problems of large raw material circulation, long reaction time, and many by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
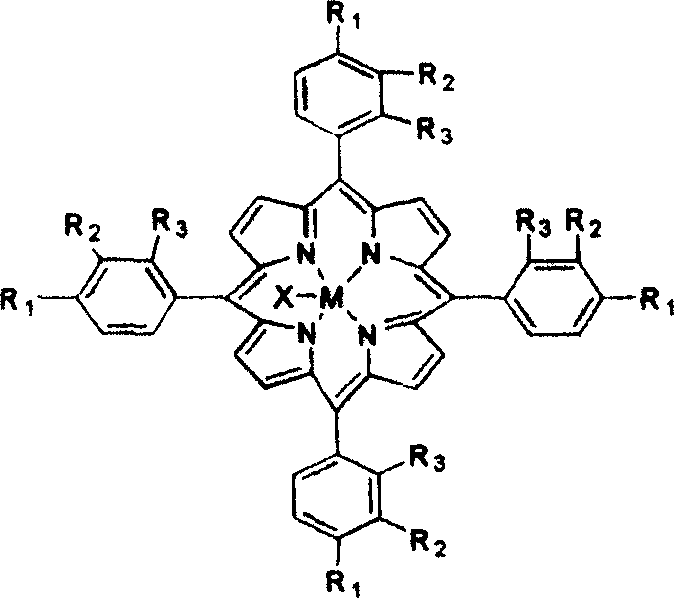
[0011] With 2mg metalloporphyrins with general formula (I) structure, R 2 =R 3 = H, R 1 =Cl, M=Mn, add 250ml of p-menthane, and pass through normal pressure air at a flow rate of 600ml / min. The reaction was stirred at 120° C. for 4 hours, and the yield of hydrogen peroxide to menthane was 15.8%. However, in the non-catalyzed oxidation process without adding metalloporphyrin under this condition, the yield of hydrogen peroxide to menthane is only 4.1%.
Embodiment 2
[0013] With 2mg metalloporphyrins with general formula (I) structure, R 2 =R 3 = H, R 1 =Cl, M=Fe, add 250ml of p-menthane, and pass air at normal pressure at a flow rate of 600ml / min. The reaction was stirred at 120° C. for 4 hours, and the yield of hydrogen peroxide to menthane was 13.6%. However, in the non-catalyzed oxidation process without adding metalloporphyrin under this condition, the yield of hydrogen peroxide to menthane is only 4.1%.
Embodiment 3
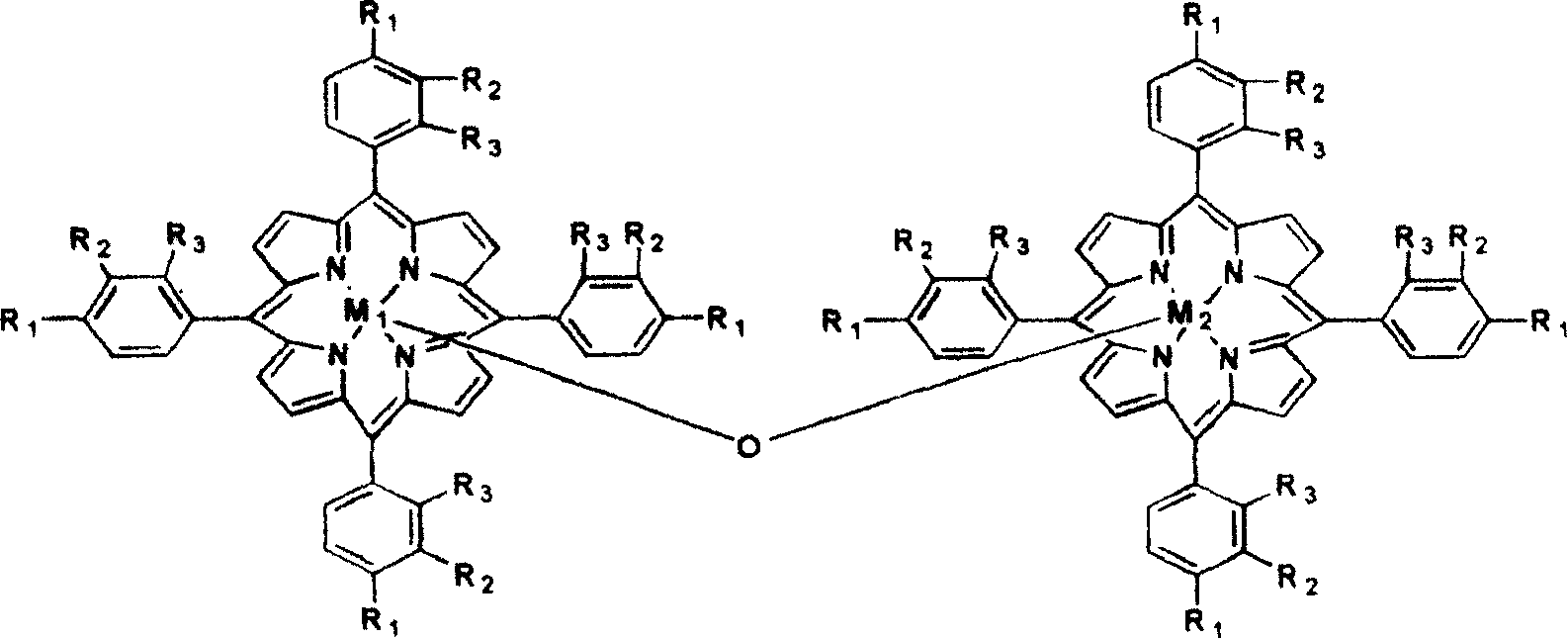
[0015] 5mg has the metalloporphyrin of general formula (II) structure, R 2 =R 3 = H, R 1 =Cl,M 1 = M 2 =Mn, 5 mg of manganese acetate was added to 250 ml of p-menthane, and air at normal pressure was fed in at a flow rate of 600 ml / min. The reaction was stirred at 120° C. for 5 hours, and the yield of hydrogen peroxide to menthane was 19.7%. However, in the non-catalyzed oxidation process without adding metalloporphyrin, the yield of menthane by hydrogen peroxide is only 5.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com