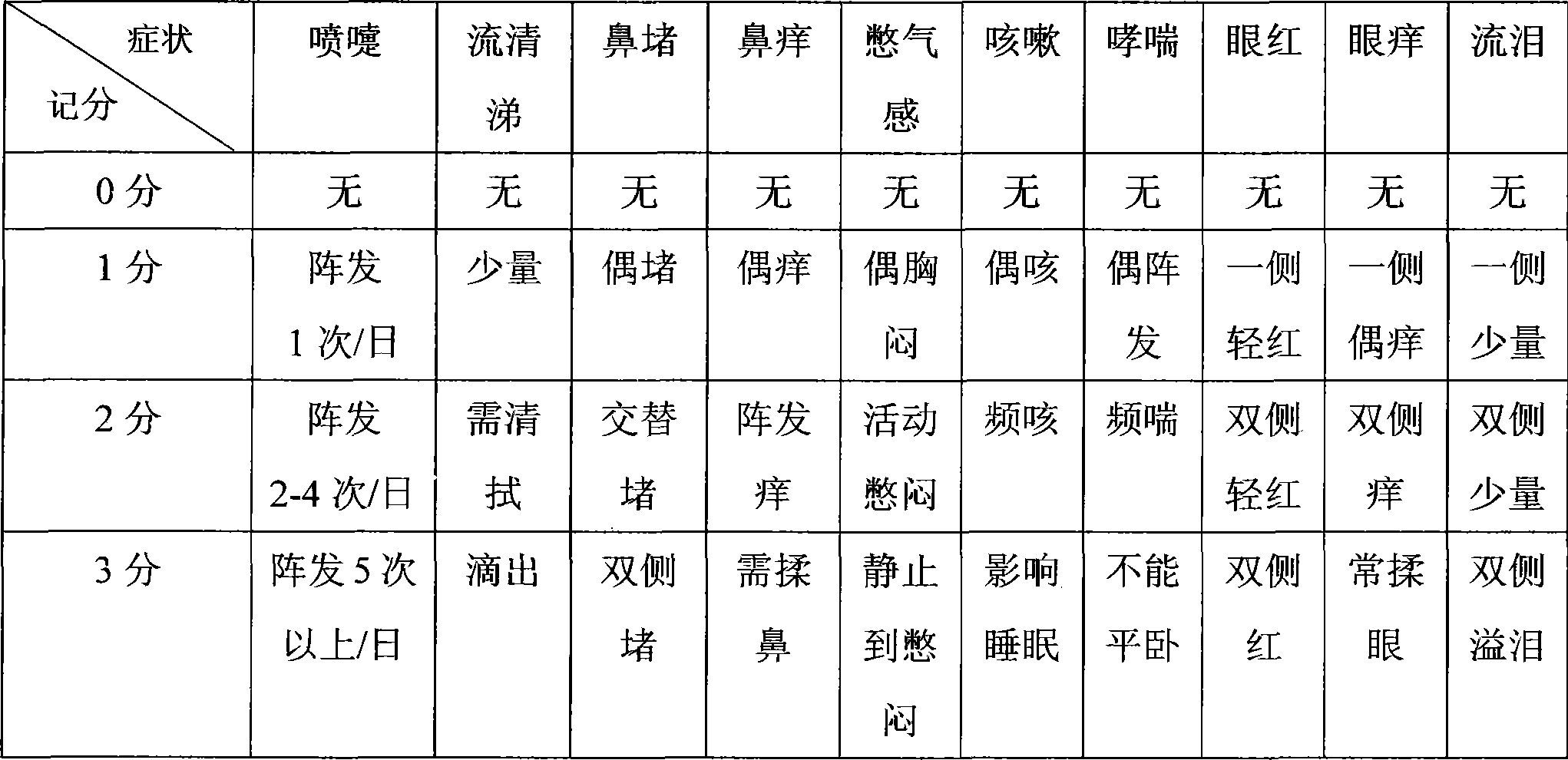
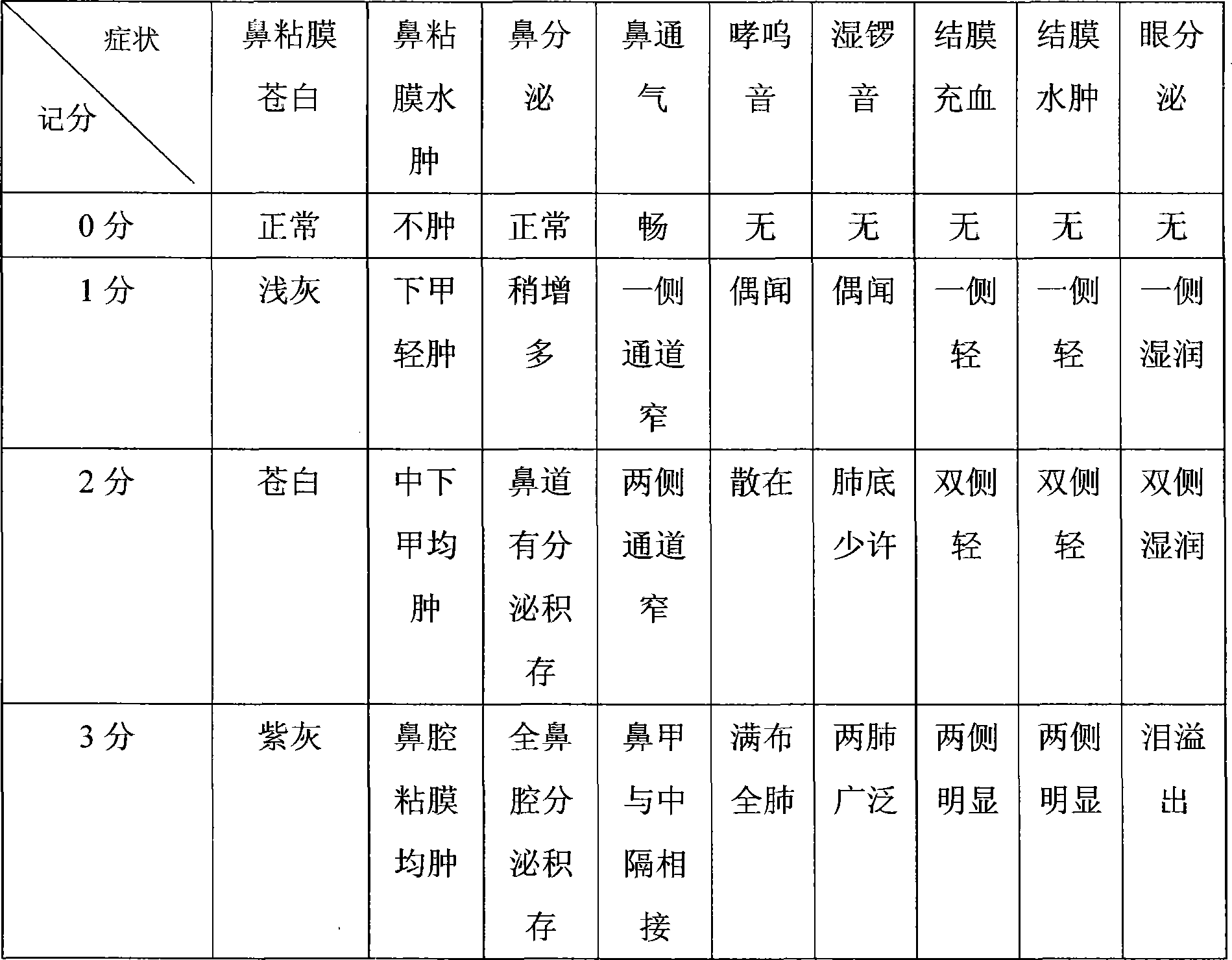
Antianaphylaxis compound containing epinastine hydrochloride
An epinastine hydrochloride and anti-allergic technology, which is applied in the direction of medical preparations containing active ingredients, drug combinations, allergic diseases, etc., can solve the problems of epinastine hydrochloride and steroids that have not been reported, and achieve anti-allergic Significant effect, reduced adverse reactions, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 Ointment preparation
[0022] The components and contents are as follows:
[0023] Epinastine Hydrochloride 0.8g
[0024] Prednisolone 0.8g
[0025] Vaseline 80g
[0026] Liquid paraffin 9g
[0027] Lanolin 8g
[0028] Pure water 1.4g
[0029] According to the above components and contents, add epinastine hydrochloride and prednisolone into pure water to dissolve, add vaseline-liquid paraffin-lanolin ointment base, mix evenly, separate bacteria, and pack separately to obtain the finished product.
Embodiment 2
[0030] Example 2 Capsule preparation
[0031] The components and contents are as follows:
[0032] Epinastine Hydrochloride 0.4g
[0033] Hydrocortisone 2.0g
[0034] Cyclodextrin 5g
[0035] Microcrystalline Cellulose 60g
[0036] Calcium hydrogen phosphate 20g
[0037] Carboxymethyl Starch Sodium 25g
[0038] Stevia 25g
[0040] Micronized silica gel 10g
[0042] The above-mentioned various raw materials are dried at low temperature, pulverized, passed through a 100-mesh fine sieve, mixed uniformly, made into a powder, and uniformly granulated by a granulator, and filled into a hollow capsule with a capsule filler after sterilization to obtain a capsule. Capsules contain 0.35 g of granules.
Embodiment 3
[0044] 1. Cases
[0045] 34 cases were verified and treated, including 9 males and 25 females, aged 12 to 63 years, with an average of 32.7 years, and the course of disease ranged from 2 to 40 years, with an average of 8.9 years. All patients had typical symptoms of bronchial asthma or allergic rhinitis. During the treatment, all patients were ineffective or unable to tolerate the treatment.
[0046] 2. Method
[0047] Oral capsules, twice a day, four capsules each time, 0.35 grams per capsule, once in the morning and before going to sleep.
[0048] The whole course of treatment for each patient was one and a half months, divided into three observation periods. The first observation period lasts for half a month. During this period, the capsules are taken orally every day according to the doctor’s advice; the second observation period also lasts for half a month, and the capsules are stopped during this period; the third observation period still lasts for half a month Durin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com