Patents
Literature
57results about How to "Has anti-allergic effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
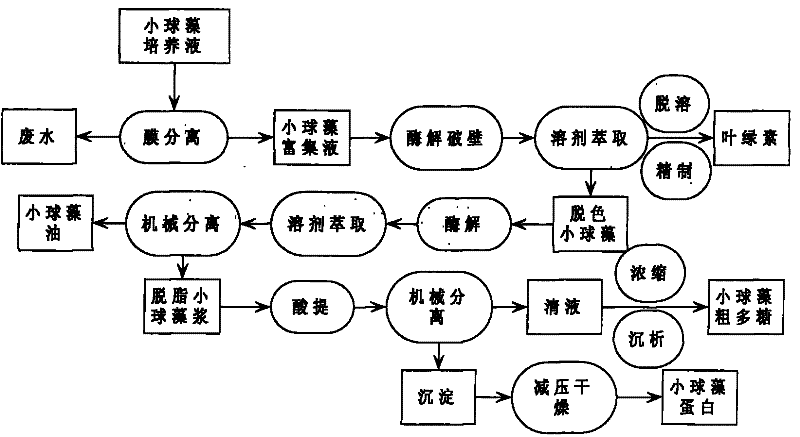
Method for continuously extracting functional components of chlorella vulgaris
InactiveCN101736045AEasy to keep activeReliable operationPeptide preparation methodsNatural dyesSolventChemistry
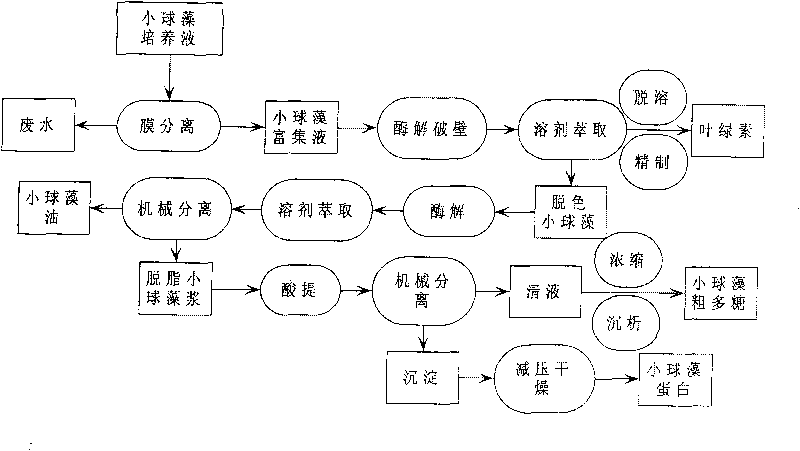
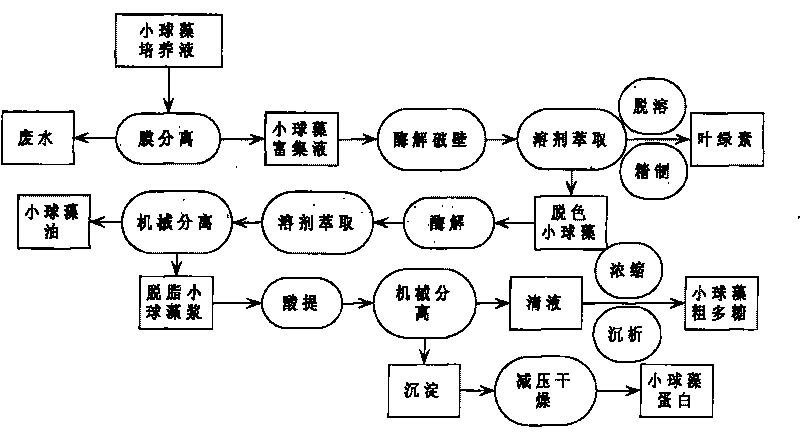
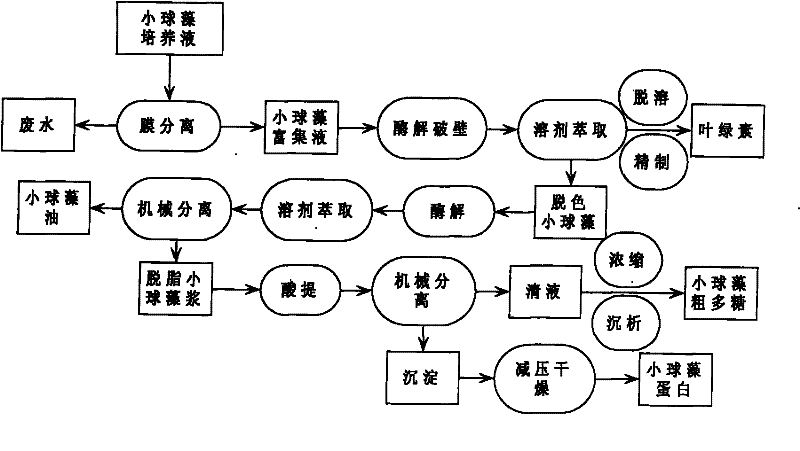
The invention relates to a method for continuously extracting functional components of chlorella vulgaris, which comprises the steps of enriching the chlorella vulgaris through the membrane separation technology, obtaining concentrated slurry of the chlorella vulgaris, adding a proper enzyme in a liquid phase system for wall-breaking, and using a solvent for extracting chlorophyll from chlorella vulgaris solution after wall-breaking, thereby being capable of obtaining a functional pigment product and reducing the color value of follow-up products; and firstly carrying out enzymatic hydrolysis on a water phase of the chlorella vulgaris after decoloring, then using the solvent for extracting functional grease, extracting active polysaccharides from the obtained degreased chlorella vulgaris by using the acid method, and then drying the other parts for obtaining crude proteins of the chlorella vulgaris. The main products comprise the chlorophyll, the active polysaccharides of the chlorella vulgaris, the functional grease of the chlorella vulgaris, the proteins of the chlorella vulgaris and the like, and the method optimizes the process on the basis of ensuring the activity of the functional components of the chlorella vulgaris, thereby obtaining high yield and reducing production cost.
Owner:BOHAI UNIV
Avocado walnut fermented soybean milk
The invention discloses avocado walnut fermented soybean milk. The fermented soybean milk is prepared by the following raw materials: by weight, 2 to 4 parts of soybeans, 1 to 2 parts of red beans, 1 to 2 parts of walnut kernels, 0.3 to 0.5 part of shelled melon seeds, 1 to 2 parts of pistachio kernels, 1 to 2 parts of corns, 70 to 80 parts of fresh milk, 2 to 4 parts of composite freeze-dried powder of fruits and vegetables, 4 to 8 parts of composite Chinese medicinal solutions, 6 to 8 parts of cane sugar, and an appropriate amount of yoghourt zymocyte. The fermented soybean milk of the invention has characteristics of a fragrant and sweet mouthfeel, unique flavor, and a blood-fat-reducing effect because of proper compatibility of composite Chinese medicinal solutions. The fruit of the avocado is high in nutritional value, and the pulp of the avocado is soft and like cheese, yellow in color, and distinctive in taste; and the fruit of avocado also contains various vitamins, abundant fat and protein, and high contents of sodium, potassium, magnesium, and calcium and the like.
Owner:BENGBU FULIN DAIRY
Bubble bath oil and preparation method thereof
InactiveCN110897937AGood skin careGood moisturizing effectCosmetic preparationsToilet preparationsGlycerolEngineering
The invention discloses bubble bath oil and a preparation method thereof. The components of the bubble bath oil comprise, by weight, 30 to 55% of sweet almond oil, 1 to 4% of cocamidopropyl DEA, 15 to25% of laurinol polyether sulfate TIPA salt, 3 to 8% of propylene glycol, 20 to 35% of lauryl alcohol polyether-4, 1 to 4% of PEG-7 glyceryl cocoate, and 0.5 to 1% of auxiliary component. The bubblebath oil has the advantages that dirt and excessive sebum on the skin can be removed; the grease balance of the skin can be maintained; the itching and dry skin state can be improved; the lubricatingfeeling of the skin can be improved; the nourishing effect is realized during cleaning; and real two-in-one effect of washing and protection is realized.
Owner:广州蜜妆生物科技有限公司
Anti-allergy soothing composition, cleansing agent comprising same and preparation method
InactiveCN108066216AHigh purityReduce sensitivityCosmetic preparationsToilet preparationsPurslane extractOfficinalis
The invention provides an anti-allergy soothing composition, a cleansing agent comprising the same and a preparation method. The anti-allergy soothing composition takes a purslane extract and a cortexmagnoliae officinalis bark extract as main raw materials, and adding PEG / PPG-25 / 30 copolymer and olive oil PEG-8 esters, wherein the purslane extract has anti-irritating, anti-allergy and soothing effects; the cortex magnoliae officinalis bark extract has anti-bacterial, anti-inflammation, anti-oxidant and anti-allergy soothing active effects; and by cooperation of the purslane extract and the cortex magnoliae officinalis extract, the anti-allergy effect can be improved. The anti-allergy soothing cleansing agent takes the anti-allergy soothing composition as a main material, and is added witha moisturizer, a softener, a cleanser and other auxiliary agents, the purslane extract and the cortex magnoliae officinalis bark extract are fused, in a using process, the cleanser can clean dirt inthe deep layer of skin, the micromolecular purslane extract and cortex magnoliae officinalis bark extract can rapidly enter the deep layer of the skin, and therefore, effects of moisturizing and relieving skin allergy are achieved.
Owner:国东启
Traditional Chinese medicine composition for treating dermatoses and preparation method thereof
InactiveCN103494891ALow costWide range of medicinesDermatological disorderPlant ingredientsSlagSalicylic acid
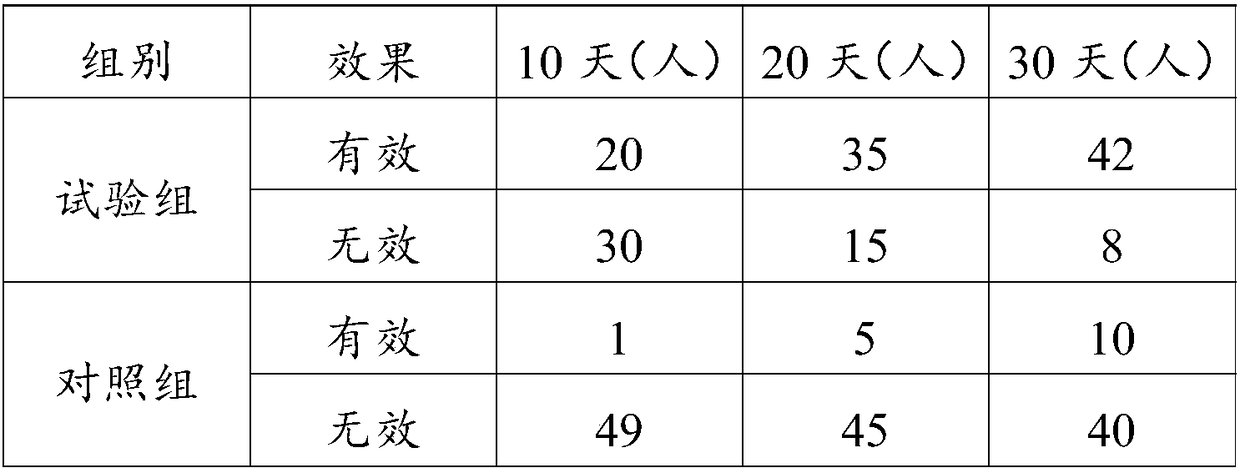
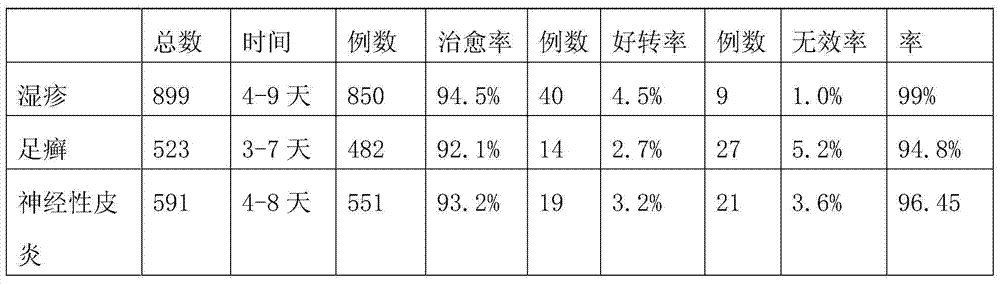
The invention discloses a traditional Chinese medicine composition for treating dermatoses and a preparation method thereof. The medicine is prepared from the following bulk drugs in parts by weight: 15-25 parts of cortex pseudolaricis, 15-25 parts of salicylic acid, 25-35 parts of fructus cnidii, and 25-35 parts of fructus kochiae. The preparation steps comprise the follows: firstly, selecting the bulk drugs in parts by weight and smashing the selected bulk drugs into fine particles; then mixing the bulk drugs which are smashed into fine particles with rice wine at 50+ / -5 degrees according to a weight proportion of 100: (250-350) for soaking for 25-30 days; then filtering away the slag to obtain a liquid medicine. The traditional Chinese medicine composition for treating dermatoses has easy source of the constituents and is low in cost. The traditional Chinese medicine composition has fast effect and high curing rate for treating the dermatoses such as eczema, tinea pedis and neurodermatitis, and also can be used for controlling relapse of the dermatoses such as eczema, tinea pedis and neurodermatitis efficiently.
Owner:韦成旺
Whitening and water replenishing complex
InactiveCN102871863BInhibition of secretionAchieve whitening effectCosmetic preparationsToilet preparationsTripropylene glycolSkin elasticity
The invention belongs to the field of cosmetics, and particularly discloses a whitening and water replenishing complex. The complex comprises from 0.05 to 0.1% of sodium hyaluronate, from 0.3 to 0.5% of dipotassium glycyrrhizinate, from 0.5 to 1% of phenethyl resorcinol, from 1 to 5% of niacinamide, from 55 to 60% of tripropylene glycol, 0.5% of phenoxyethanol, from 0.02 to 0.05% of glycolic acid, from 0.05 to 0.1% of sodium bisulfite and from 32.75 to 41.58% of deionized water. The whitening and water replenishing complex is directly added into a whitening and water replenishing moisturizing product at the temperature lower than 50 DEG C and is uniformly stirred. The whitening and water replenishing complex realizes a whitening effect, and can suppress melanin secretion of melanocyte simulating cells of the surface of the skin of a user, so that the skin of the user is whitened. Besides, the whitening and water replenishing complex realizes an anti-wrinkle effect, can promote collagen and elastin secretion of fibroblasts and realizes effects of preventing ageing of the skin of the user and improving elasticity of the skin of the user.
Owner:CHENGDU DAME GREEN COMMODITY
Method for preparing soymilk powder contg. micron additives for tonifying-Yin and eliminating-fatigue
InactiveCN1792188APlay a role in health careGood health effectMilk preparationUnknown materialsBiotechnologyAdditive ingredient
A micron-class health-care soybean milk powder for nourishing Yin, relieving fatigue and improving immunity is prepared from traditional soybean milk powder, the micron-particles of pilose asiabell root, lily bulb, turtle shell, cactus and dendrobium, and the oligose, useful intestinal bacteria and freeze-dried royal jelly. It has rich nutrients and high effect.
Owner:余内逊
Cosmetic additive as well as preparation method thereof and cosmetic containing same
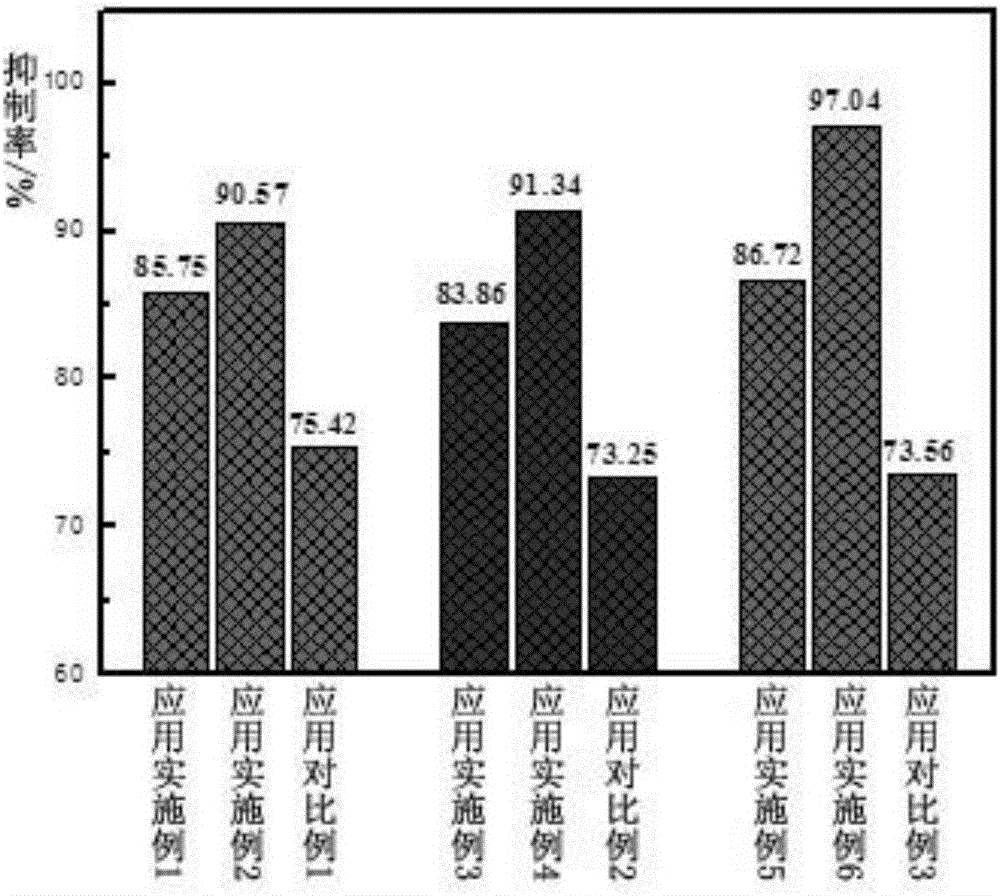
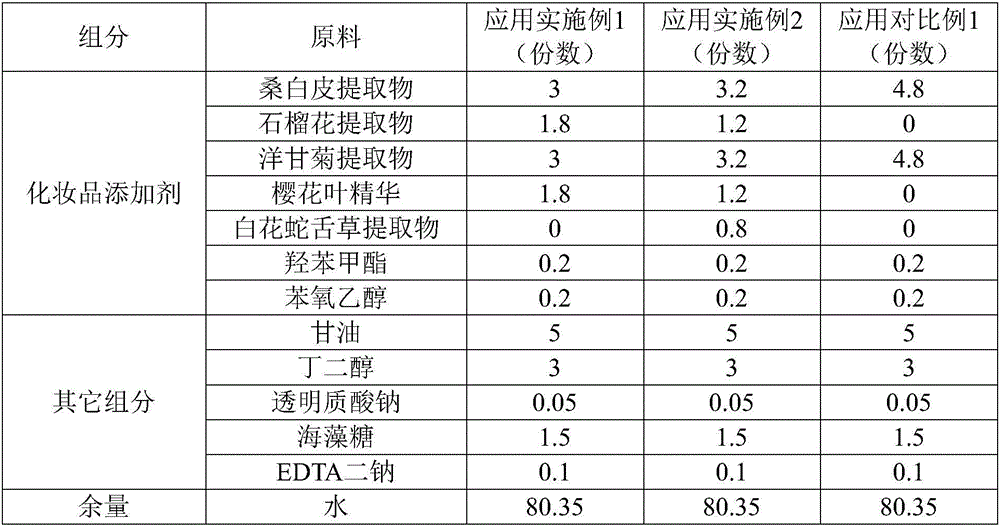
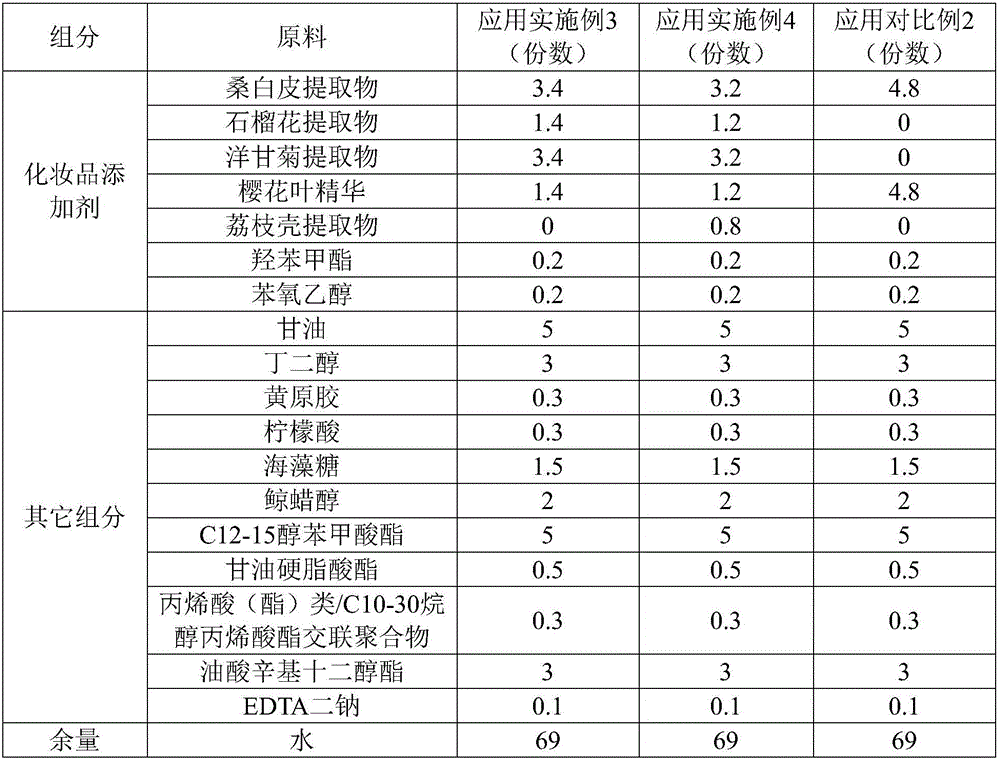
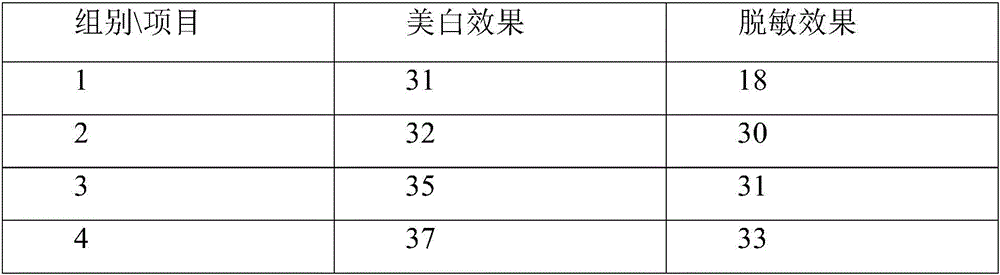
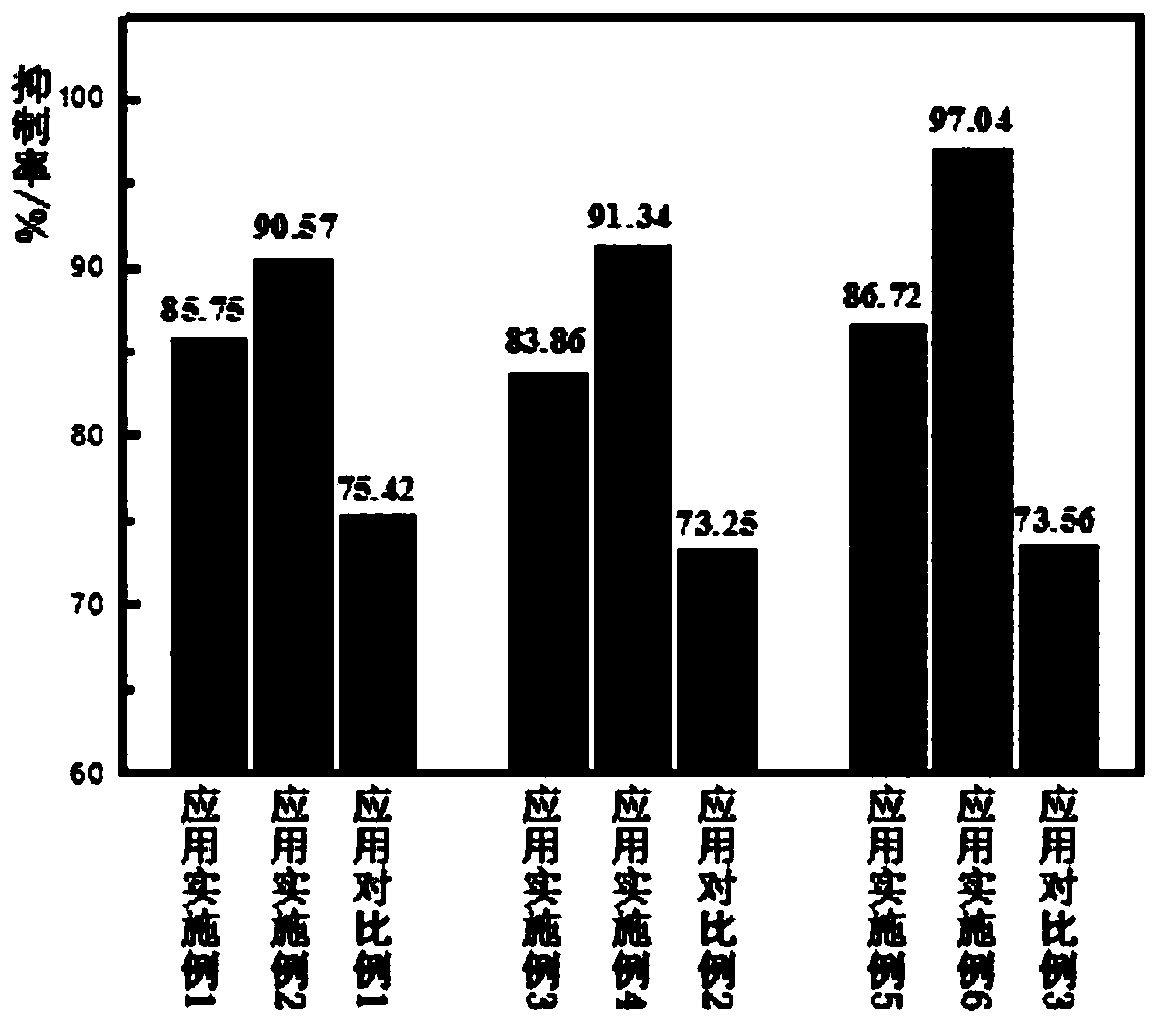
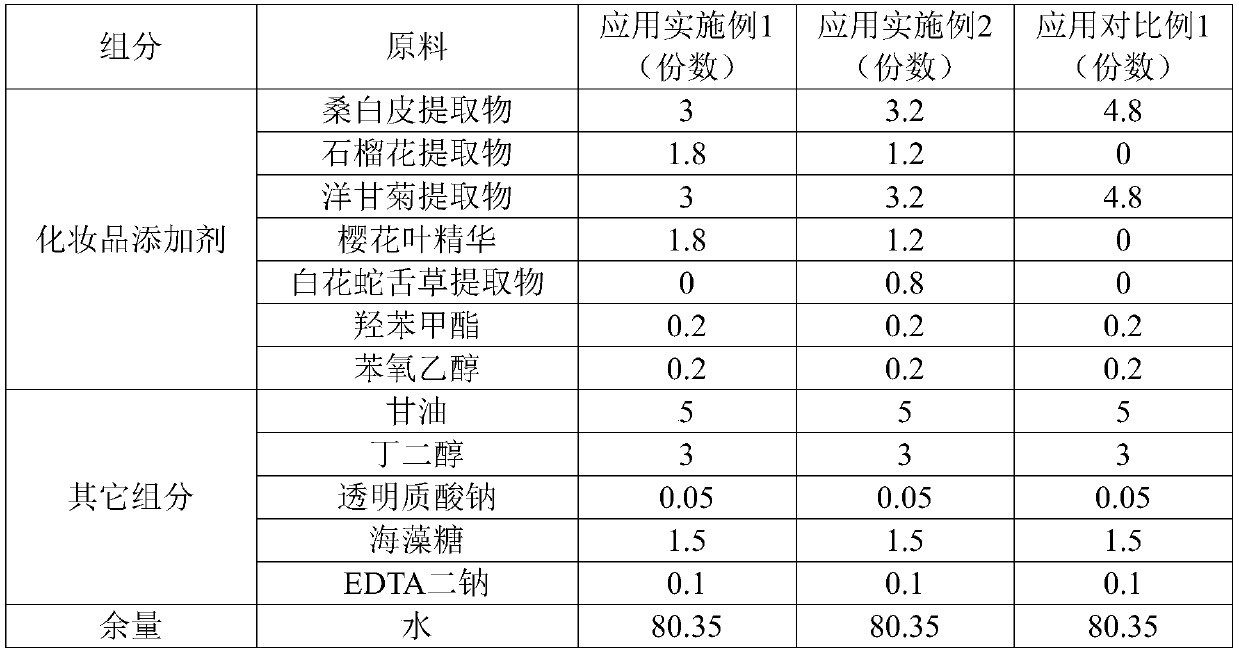
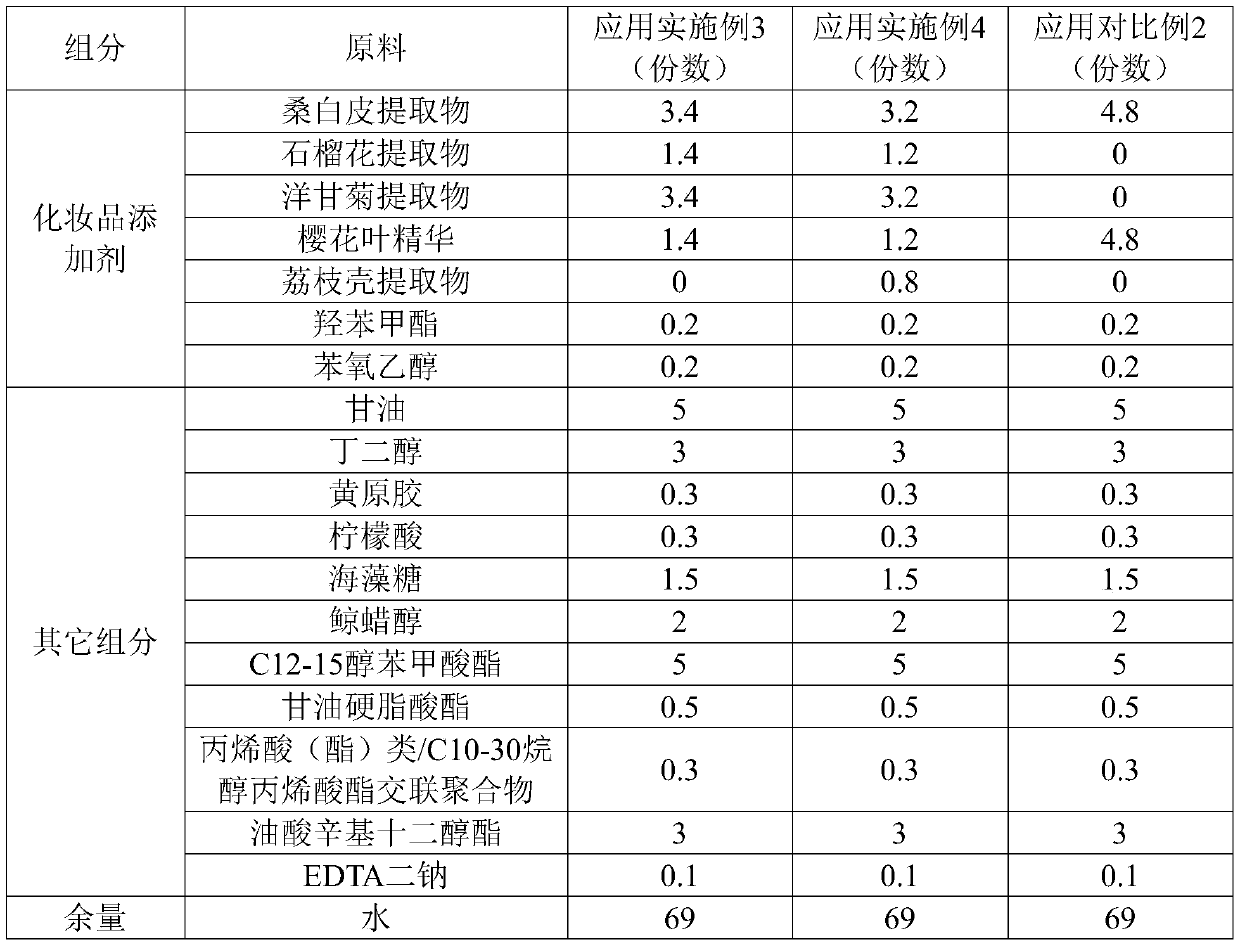
ActiveCN106726923AGood whitening effectHas anti-allergic effectCosmetic preparationsToilet preparationsChamomile extractMelanin
The invention provides a cosmetic additive as well as a preparation method thereof and a cosmetic containing the same. The cosmetic additive comprises white mulberry root-bark extract, pomegranate flower extract, chamomile extract and oriental cherry leaf essence. According to the cosmetic additive provided by the invention, all components are plant extracts which are safe and little irritant; and moreover, the melanin formation can be inhibited in different stages, a synergistic effect is realized on melanin inhibition, the melanin deposition is slowed down, and a whitening effect is improved. The cosmetic additive provided by the invention also has an anti-allergic effect.
Owner:GUANGZHOU KENENG COSMETICS RES CO LTD +1
Medical application preventing and curing phlebitis and preparing method thereof
InactiveCN105147726AInhibit aggregationReduce inflammationOrganic active ingredientsInorganic boron active ingredientsIndwelling cathetersMultiple component
The invention discloses medical application preventing and curing phlebitis and a preparing method thereof. The application is prepared from, by weight, 3-10% of alginate, 3-4% of aloe pectin, 35-55% of magnesium sulfate, 1-2% of cetirizine, 1-2% of clarityne, 3-7% of wet storage agent, 1-3% of sodium tetraborate decahydrate and the balance deionized water. The multiple components in the application have the synergistic effect, inflammatory cells are restrained from gathering on the inflammation part, inflammatory responses are reduced, and the inflammation diminishing and swell stopping efficiency is high; the application is used for maintaining a PICC indwelling catheter, the occurrence rate of mechanical phlebitis is obviously reduced, the effect of treating the phlebitis is remarkable, onset time is short, and the swelling subsidence rate is high; the application has the effects of expanding the blood vessel and promoting blood circulation. The application is convenient to use and easy to operate, and relieves pain of patients.
Owner:尹楠楠
Fig and vine root beauty-maintaining and young-keeping crystal and preparation method thereof
InactiveCN102150917AMaintain skin elasticityInhibits the formation of melaninFood preparationCistancheTapioca starch
The invention discloses a fig and vine root beauty-maintaining and young-keeping crystal and a preparation method thereof, relating to a beauty-maintaining and young-keeping crystal and a preparation method thereof. The fig and vine root beauty-maintaining and young-keeping crystal is prepared from fig, carrot, dried vine root, soybean, mung bean, pea, dried Chinese yam, dried cucumber, dried lichee, agaric, lentinus edodes, grape pip, cistanche, glossy privet fruit, leonurus, prepared rehmannia root, tuber fleeflower root, licorice root, tapioca starch, dried potato powder, granulated sugar and malt sugar. The fig and vine root beauty-maintaining and young-keeping crystal has the effects of regulating kidney yin and kidney yang, maintaining beauty and keeping young, and does not contain any preservative and chemical addition agent.
Owner:郑延明
Handmade soap containing samara oil and oat beta-glucan
InactiveCN106967541AHigh antioxidant capacityHighly AntioxidantSoap detergents with organic compounding agentsDetergent compounding agentsAqueous solutionNatural aging
The invention provides handmade soap containing samara oil and oat beta-glucan. The handmade soap comprises the samara oil, grease, sodium hydroxide, the oat beta-glucan, samara honey, plant essence and deionized water. A production method of the handmade soap includes: sufficiently dissolving the sodium hydroxide into the deionized water or milk to obtain an alkaline solution; heating the grease to 45 DEG C, then adding the alkaline solution into the grease while stirring, and constantly stirring until the alkaline solution and the grease are evenly mixed and the mixture is thick to obtain a soap base; adding the an oat beta-glucan solution, a samara honey aqueous solution and the plant essence into the soap base to obtain finished-product soap liquid; pouring the soap liquid into a mould, and demoulding; performing soap ripening for 1-6 months after the demoulding. The handmade soap has the advantages that the samara oil is compounded with the oat beta-glucan, the samara oil and the oat beta-glucan are simultaneously added into handmade soap to achieve a synergic effect, and the produced handmade soap is fine and smooth and rich in foam, capable of thoroughly eliminating greasy dirt deep in pores, capable of delaying the natural aging of the skin and capable of allowing the skin to be moist, glossy and rich in elasticity.
Owner:SHANXI LONGSHUI BIOLOGICAL TECH
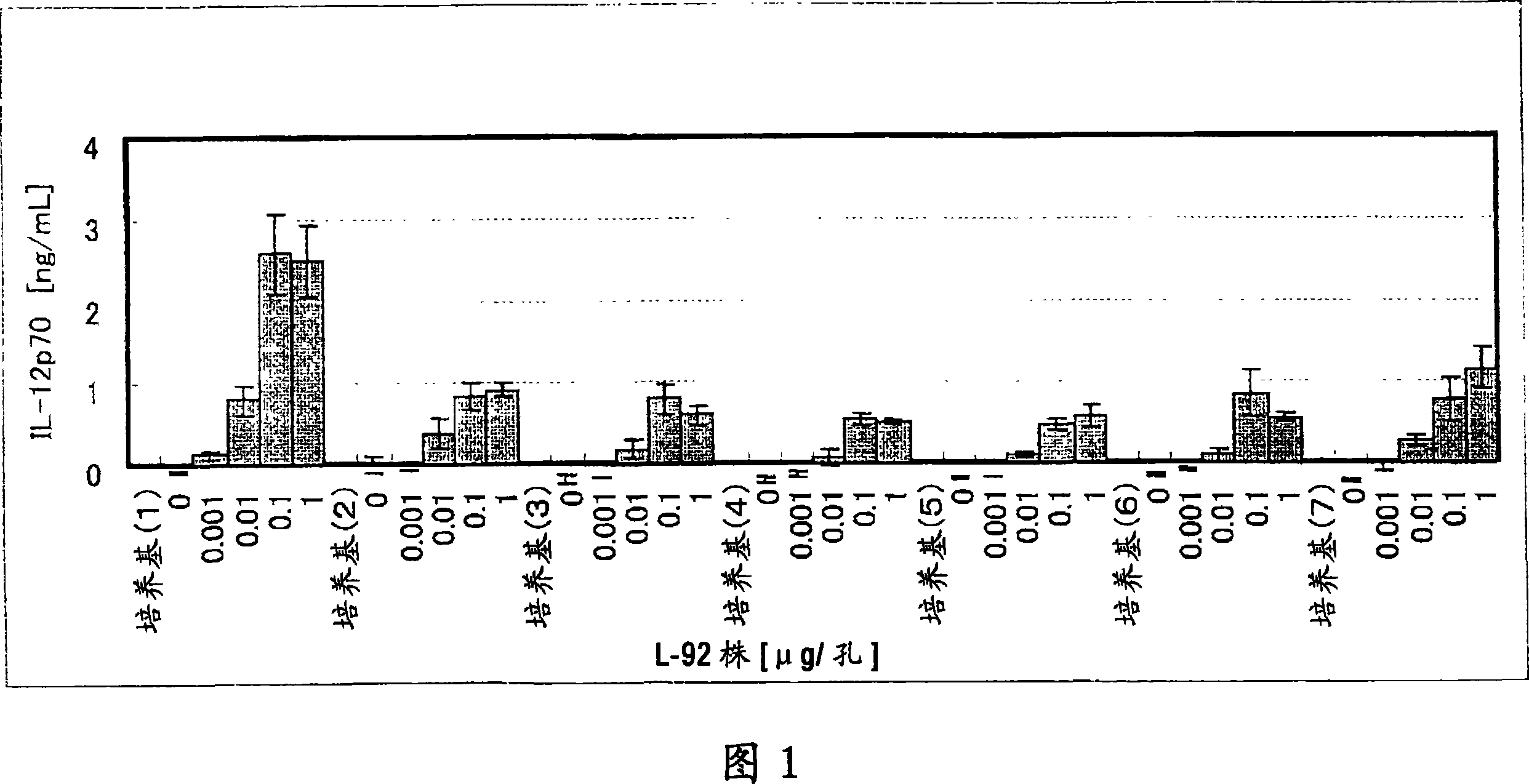
Method of producing lactic acid bacterium having antiallergic effect
InactiveCN101098957AHas anti-allergic effectImprove convenienceBacteriaBacteria material medical ingredientsLactic acid bacteriumActive ingredient
It is intended to disclose a method of producing a lactic acid bacterium which has an antiallergic effect characterized by comprising culturing a lactic acid bacterium in a medium containing a casein hydrolysate. Furthermore, it is intended to provide an antiallergic agent containing, as the active ingredient, a lactic acid bacterium having been cultured by the method as described above; and a food and a drink having an antiallergic effect which contain, as the active ingredient, a lactic acid bacterium having been cultured by the method as described above.
Owner:CALPIS
Tooth whitening gel and preparation method and application thereof
InactiveCN106344432AHave a whitening effectReached dental cariesCosmetic preparationsToilet preparationsMentholStaining
The invention provides tooth whitening gel. The gel comprises the following components in parts by weight: 4-8 parts of an oxidizing agent, 1.5-5.5 parts of thickening aids, 30-40 parts of deionized water, 45-55 parts of glycerin, 0.01-0.5 part of menthol, 1-5 parts of xylitol and 0.5-4 parts of triethanolamine. The invention also provides a method for preparing the tooth whitening gel. The method comprises the following steps: sequentially pouring the oxidizing agent, thickening aids, deionized water, glycerin, menthol and xylitol into a container on an assembly line by adopting a perfusion method, sealing the container after all the components are completely poured, standing under the condition of the temperature of 10-20 DEG C for 20-30 hours, and molding. The invention also relates to application of the tooth whitening gel in improvement of tooth staining, wherein the tooth staining comprises extrinsic staining and intrinsic staining.
Owner:长沙蒽俏蔓生物科技有限公司
A method for continuous extraction of chlorella functional components
InactiveCN101736045BIncrease concentrationNo pollution in the processPeptide preparation methodsNatural dyesSlurrySolvent
A method for continuous extraction of functional components of chlorella, in which chlorella is enriched by membrane separation technology to obtain concentrated chlorella slurry, and appropriate enzymes are added to the liquid phase system to break the wall, and the broken chlorella Chlorella solution extracts chlorophyll with a solvent to obtain functional pigment products and reduce the color value of subsequent products; the decolorized chlorella is first enzymatically hydrolyzed in the water phase, and then the functional oil is extracted with a solvent to obtain defatted chlorophyll The active polysaccharides of the algae are extracted by an acid method, and the rest is dried to obtain the crude protein of the algae. The main products include chlorophyll, chlorella active polysaccharides, chlorella functional oils, chlorella protein, etc. On the basis of ensuring the activity of chlorella functional components, the process is optimized to obtain a higher yield and reduce Cost of production.
Owner:BOHAI UNIV
Drug composition of amoxicillin potassium clavulanate and application thereof
ActiveCN106474122AElevated serum IgEEnhanced inhibitory effectAntibacterial agentsAntisepticsAir pollutionPotassium Clavulanate
The invention provides a drug composition, wherein the ratio of amoxicillin and potassium clavulanate in the drug composition is 7: 1. The drug composition can significantly lighten the rise of IgE level induced by allergen, and can be used for preventing or treating asthma, in particular to the asthma caused by air pollution such as haze; besides, the drug has good effect for the asthma combined bacterial infection.
Owner:XIANGBEI WELMAN PHARMA CO LTD +1
Moisturizing skin care composition and moisturizing skin care lotion
InactiveCN108685787APenetrate fastImprove moisturizing functionCosmetic preparationsToilet preparationsCentella asiatica extractMedicine
The invention relates to a moisturizing skin care composition and a moisturizing skin care lotion. The moisturizing skin care composition provided by the invention is prepared from a substrate, and the following components in percentage by weight: 0.5 to 1.5 percent of cordyceps sinensis extract; 0.1 to 0.5 percent of centella asiatica extract; 0.1 to 0.3 percent of portulaca oleracea extract; 0.1to 0.3 percent of vitex agnus castus extract; 0.1 to 0.3 percent of chamomilla recutita flower extract; 0.3 to 0.7 percent of ganoderma lucidum stem extract; 0.3 to 0.7 percent of nopal cactus fruitextract; 0.3 to 0.7 percent of ginseng root extract. The raw materials of the moisturizing skin care lotion provided by the invention are prepared from the moisturizing skin care composition, a moisturizer, a conditioner, a thickener, a chelating agent, and a functional aid. The moisturizing skin care composition and the moisturizing skin care lotion have deep moisturizing and anti-aging effects,and are mild and non-irritating to the skin.
Owner:广州巨蔻化妆品有限公司
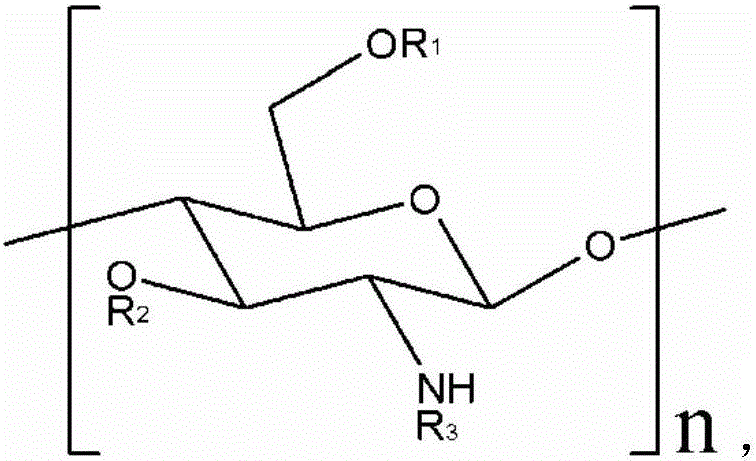
Applications of carboxymethyl chitosan to preparation or screening of products for antiallergic treatment
ActiveCN106581039AHas anti-allergic effectOrganic active ingredientsImmunological disordersActive componentMedicine
The invention discloses applications of carboxymethyl chitosan to preparation or screening of products for antiallergic treatment. Cells and human body tests show that carboxymethyl chitosan has antiallergic efficacy and can be used as an antiallergic active component which is added into the product with practical application values.
Owner:SHANGHAI TAO SHENG BIOTECH CO LTD
Moisturizing skin care composition and moisturizing skin care water
InactiveCN108685786AHas anti-allergic effectEfficient removalCosmetic preparationsToilet preparationsMoisturizerGANODERMA LUCIDUM STEM
The invention relates to a moisturizing skin care composition and moisturizing skin care water. The moisturizing skin care composition provided by the invention is prepared from a substrate, and the following components in percentage by weight: 0.1 to 1 percent of cordyceps sinensis extract; 0.01 to 0.2 percent of ganoderma lucidum stem extract; 0.01 to 0.2 percent of nopal cactus fruit extract; 0.01 to 0.2 percent of ginseng root extract. The raw materials of the moisturizing skin care water provided by the invention are prepared from the moisturizing skin care composition, a moisturizer, a conditioner, a thickener, a chelating agent, and a functional aid. The moisturizing skin care composition and the moisturizing skin care water have deep moisturizing effects, and are mild and non-irritating to the skin.
Owner:广州巨蔻化妆品有限公司
Multi-effect toothpaste
InactiveCN105213263AReduce stimulationHas anti-allergic effectCosmetic preparationsToilet preparationsFoaming agentSide effect
The invention discloses multi-effect toothpaste. The multi-effect toothpaste comprises the following components in parts by mass: 3 to 8 parts of abrasive agent, 2 to 7 parts of sweetening agent, 1.5 to 3 parts of essence, 6 to 21 parts of eritrichium deltodentum, 2 to 5 parts of washing foaming agent, 0.8 to 1.2 parts of adhesive, 1 to 3 parts of pepperweed seed, 2 to 8 parts of moisture preserving agent, 2 to 8 parts of paeonol, 6 to 10 parts of honeysuckle extract and 3 to 8 parts of sweet tea extract. The multi-effect toothpaste has an anti-allergic effect and also has the effects of removing halitosis and oral bacteria, has a variety of functions, and does not have a side effect.
Owner:CHENGDU SHUNFA DISINFECTANT & WASHING TECH
Plant source deodorization spray and application thereof
InactiveCN105920987ABactericidalHas anti-allergic effectDispersed particle separationDeodrantsAlcoholDistilled water
The invention discloses a plant source deodorization spray. The plant source deodorization spray is prepared from, by weight, 50-60 parts of 95% alcohol, 25-35 parts of plant combined extracting solution and 20-30 parts of distilled water. The plant combined extracting solution is a combined solution of a dianthus chinensis extracting solution, a sculellaria barbata extracting solution, a polygala tenuifolia extracting solution, a white birch extracting solution and a cortex albiziae extracting solution. The invention further discloses a method for dissolving stink through the plant source deodorization spray. According to the method, the stink is pumped through a sucking pump, dilution is conducted 2-4 times, and then the plant source deodorization spray is used for carrying out continuous spraying for 3-8 h. The plant source deodorization spray has the advantages that the new plant source deodorization spray is developed, cost is low, consumed time is short, the deodorization effect is good, bacteria can be restrained and killed, and the spray is safe and free of toxins, and does not generate secondary pollution to the environment.
Owner:FOSHAN JUCHENG BIOCHEM TECH RES & DEV CO LTD
Fig-jujube drink for preventing anaphylaxis and preparation method thereof
The invention discloses a fig-jujube drink for preventing anaphylaxis and a preparation method thereof, relating to a drink for preventing anaphylaxis and a preparation method thereof. The fig-jujube drink is prepared from raw materials such as dried figs, big dried jujubes, propolis, colla asini, ginkgo, dried carrot slices, barbary wolfberry fruits, desertliving cistanche, glossy privet fruits, tuber fleeceflower roots, white hyacinth beans, licorice roots, granulated sugar, protein candies, honeys, citric acids and yeasts through processing. The fig-jujube drink disclosed by the invention can be used for edible and pharmaceutical purposes, and has an anaphylaxis control function as well as the advantages of fatigue resistance, aging resistance, tumor prevention and rich nutrition.
Owner:郑延明
Cosmetic additive, preparation method thereof, and cosmetic containing the cosmetic additive
ActiveCN106726923BGood whitening effectHas anti-allergic effectCosmetic preparationsToilet preparationsBiotechnologyPunica granatum flower extract
The invention provides a cosmetic additive as well as a preparation method thereof and a cosmetic containing the same. The cosmetic additive comprises white mulberry root-bark extract, pomegranate flower extract, chamomile extract and oriental cherry leaf essence. According to the cosmetic additive provided by the invention, all components are plant extracts which are safe and little irritant; and moreover, the melanin formation can be inhibited in different stages, a synergistic effect is realized on melanin inhibition, the melanin deposition is slowed down, and a whitening effect is improved. The cosmetic additive provided by the invention also has an anti-allergic effect.
Owner:GUANGZHOU KENENG COSMETICS RES CO LTD +1
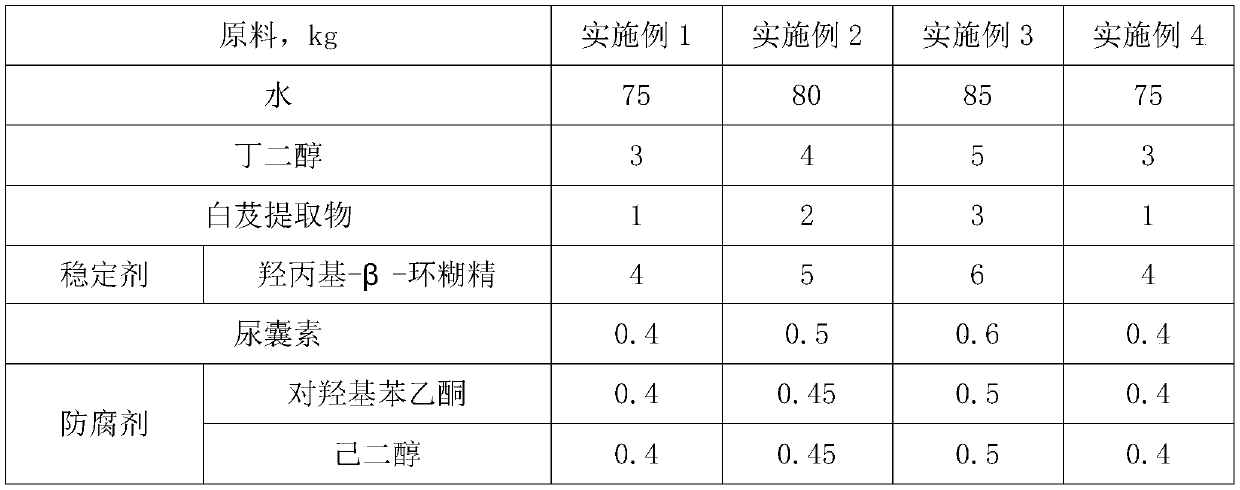
Bletilla striata gelatin repairing emulsion and preparation and application methods thereof
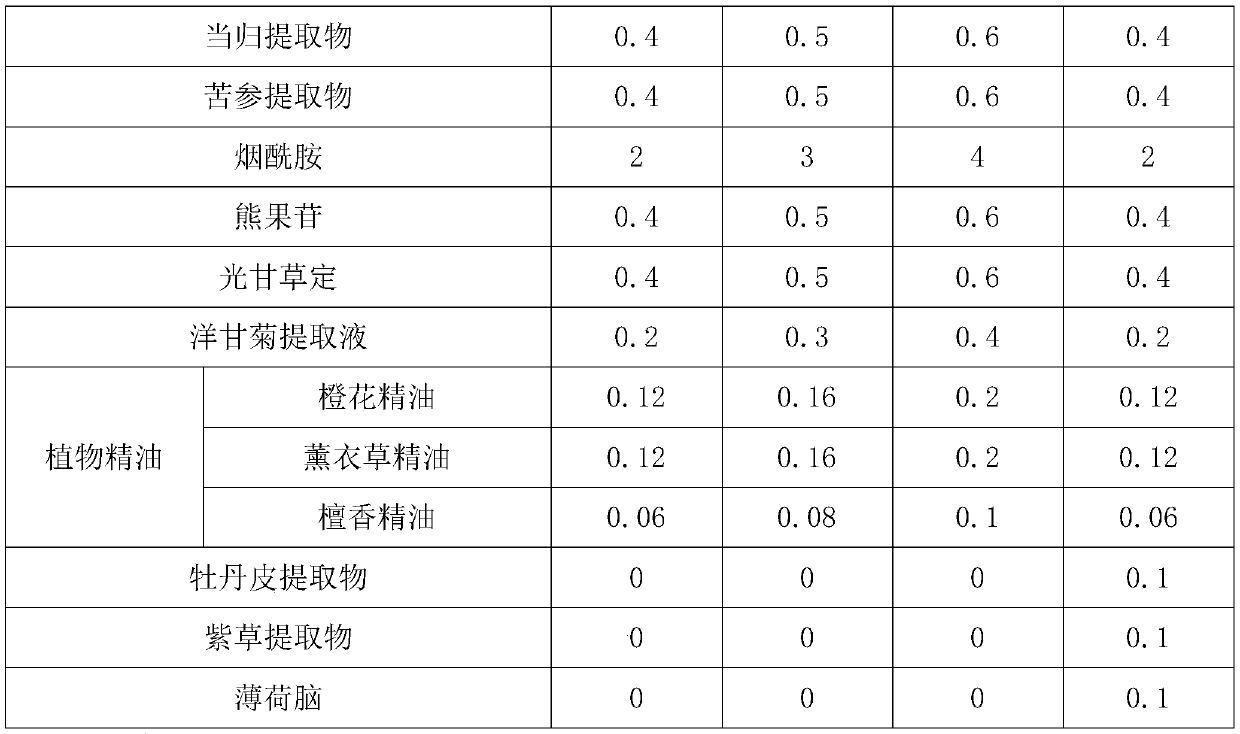
InactiveCN110269830ARevitalize new lifeLighten acne marksCosmetic preparationsToilet preparationsBletilla striataNicotinamide
The invention discloses a bletilla striata gelatin repairing emulsion and preparation and application methods thereof and belongs to the technical field of cosmetics. The bletilla striata gelatin repairing emulsion is characterized by being prepared from, by weight, 75-85 parts of water, 3-5 parts of butanediol, 1-3 parts of a bletilla striata extract, 4-6 parts of a stabilizer, 0.4-0.6 part of allantoin, 0.8-1 part of a preservative, 0.4-0.6 part of a radix angelicae sinensis extract, 0.4-0.6 part of a radix sophorae flavescentis extract, 2-4 parts of nicotinamide, 0.4-0.6 part of arbutin, 0.4-0.6 part of glabridin, 0.2-0.4 part of a chamomile extraction solution and 0.3-0.5 part of plant essential oil. The bletilla striata gelatin repairing emulsion has the advantages of fading scars, relieving the itch, diminishing inflammation, relieving pain and relieving the mood.
Owner:浙江高妍科技有限公司
Tea for reducing blood fat and nourishing skin and preparation method thereof
The invention provides a tea for reducing blood fat and nourishing skin and a preparation method thereof and relates to a healthcare food for reducing blood fat. The method comprises the following steps: by taking puer tea, roxburgh rose and red dates as raw materials, smashing; breaking the cells; mixing; and drying. In a preparation process, the powder of the raw materials is more fine and smooth; the loss of raw material components is reduced; the taste of the raw materials and the brewing measurement are promoted; the tea can be easily absorbed by human body; the effects of reducing blood fat and nourishing skin are better.
Owner:厦门特旭生物科技有限公司
Method for preparing milk powder contg. micron additives for tonifying-Yin and eliminating-fatigue
InactiveCN1792189APrevent proliferationWith sedationMilk preparationUnknown materialsBiotechnologyAdditive ingredient
A micron-class health-care milk powder for nourishing Yin, relieving fatigue and improving immunity is prepared from traditional milk powder, the micron-particles of pilose asiabell root, lily bulb, turtle shell, cactus and dendrobium, and the oligose, useful intestinal bacteria and freeze-dried royal jelly. It has rich nutrients and high effect.
Owner:余内逊
Anti-allergy composition containing ilex cornuta leaf extract and application thereof
InactiveCN108283598AResistance to intrusionGood immune regulationCosmetic preparationsAntipyreticIlex cornutaLonicera hypoglauca
The invention discloses an anti-allergy composition containing an ilex cornuta leaf extract. The anti-allergy composition comprises the following components in parts by weight: 0.1-10 parts of a glycyrrhiza glabra leaf extract, 0.1-10 parts of the ilex cornuta leaf extract, 0.1-10 parts of a lonicera hypoglauca extract, and 0.1-10 parts of a hypericum perforatum extract. The glycyrrhiza glabra leaf extract, the ilex cornuta leaf extract, the lonicera hypoglauca extract, and the hypericum perforatum extract are subjected to complex formulation and synergistic interaction, the anti-allergy composition has good anti-allergy efficacy, increases the human immunoloregulation capability, and improves the tolerance of sensitive skin.
Owner:FOSHAN JIAOFU BIOTECH CO LTD
Face cleansing mousse and preparation method thereof
ActiveCN109316369ADense foamGood foam durabilityCosmetic preparationsToilet preparationsChemistryStearic acid
The invention discloses face cleansing mousse and a preparation method thereof. The face cleansing mousse comprises a phase A, a phase B and a phase C, wherein the phase A is prepared from the following various ingredients in percentage by weight: 5-15% of potassium cocoyl glycinate, 2-6% of cocoalkanoylamido propyl betaine, 2-5% of a fatliquoring agent, 0.5-3% of lauric acid, 0.5-2% of myristic acid and 0.5-2% of stearic acid; the phase B is prepared from the following various ingredients in percentage by weight: 2-5% of glycerine, 0.5-1% of povidone, 0.2% of disodium EDTA, 0.5% of dipotassium glycyrrhizinate, 0.5% of panthenol and the balance of water; the phase C is prepared from the following various ingredients in percentage by weight: 0.4% of a compound preservative and 0.8% of a combination preservative; the sum total of the mass percents of the various ingredients in the phase A, the phase B and the phase C is 100%. The face cleansing mousse product can lower the discomfort ofsensitive skin, has very little residue on the skin and can promote the skin barrier for skin reestablishment.
Owner:德国欧悦安股份有限公司
Traditional Chinese medicine composition for treating allergic skin diseases and preparation method thereof
InactiveCN107714977ANo side effectsSuitable for a wide range of peopleImmunological disordersDermatological disorderPeppermintsCamellia nitidissima
The invention relates to the technical field of traditional Chinese medicines, in particular to a traditional Chinese medicine composition for treating allergic skin diseases. The traditional Chinesemedicine composition is prepared from the following raw materials in parts by weight: 40-60 parts of camellia nitidissima, 15-20 parts of peppermint, 20-30 parts of apricot seed, 20-40 parts of fructus forsythiae, 10-15 parts of reed rhizome and 10-15 parts of licorice root. The traditional Chinese medicine composition provided by invention effectively gives play to medicinal values of traditionalChinese medicines through reasonable compatibility of a plurality of traditional Chinese medicines, and can be used for treating allergic skin diseases.
Owner:DALIAN UNIVERSITY
Triamcinolone acetonide acetate injection and preparation method thereof
InactiveCN104414972AImprove securityReduce adverse reactionsOrganic active ingredientsAntipyreticTRIAMCINOLONE ACETONIDE INJECTIONCellulose
The invention discloses a triamcinolone acetonide acetate injection and a preparation method thereof. The injection is prepared from 10.0g of triamcinolone acetonide acetate, sodium chloride which is 5.00-20 percent of the injection in a weight-to-volume ratio, sodium merthiolate which is 0.005-0.01 percent of the injection in a volume ratio, HS-15 which is 1.00-15 percent of the injection in a volume ratio, sodium carboxymethylcellulose which is 10.00-30 percent of the injection in a volume ratio and the balance of water for injecting. The preparation method comprises the following steps: putting triamcinolone acetonide acetate, sodium merthiolate, sodium chloride and sodium carboxymethylcellulose in a beaker according to the prescription, adding partial water for injecting, uniformly stirring, adding activated carbon, uniformly stirring, and filtering to obtain a solution (1); weighing a proper amount of water for injecting at 30-40 DEG C, adding HS-15 which is 1.00-15 percent of the injection in a volume ratio, and uniformly stirring to obtain a solution (2); mixing the solution (1) and the solution (2), uniformly stirring, adding the water for injecting to total amount, regulating the pH value of the mixture to be 5.5-7.0 by using acetic acid, filtering and filling and sealing. By adopting a brand-new prescription and process, a produced product has stable quality, and the safety of clinical administration can be remarkably improved.
Owner:CHENGDU LIST PHARMA
Novel toothpaste with effect of treating decayed teeth
InactiveCN109833245AWith dewormingHas anti-allergic effectCosmetic preparationsToilet preparationsPhosphateGlycerol
The invention belongs to the technical field of toothpaste, and in particular, relates to a novel toothpaste with the effect of treating decayed teeth. The novel toothpaste with the effect of treatingthe decayed teeth is prepared from the following raw materials. The toothpaste comprises mixed powder prepared from 2-4 g of green tea powder, 3-5 g of lemon powder, 1-5 g of chamomile powder, 2-6 gof orange peel powder, 3-6 g of wild chrysanthemum powder and 5-7 g of liquorice powder and having the particle size of nanoscale. The toothpaste also comprises 25-35 g of glycerol, 7-12 g of lauryl mercaptan, 3-6 g of sodium alkyl benzene sulfonate, 10-15 g of ethylene glycol, 0.05-1.0 g of potassium dihydrogen phosphate, 3-6 g of granulated sugar, 0.5-1.6 g of a pigment, 0.5-2.5 g of lemon essence, and the balance being purified water. The novel toothpaste with the effect of treating the decayed teeth has a certain anti-allergic effect, and has the advantages of insect removal, traditional Chinese medicine conditioning, inflammation diminishing, bacteria resistance and health care of teeth.
Owner:沈阳华企力拓企业服务有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com