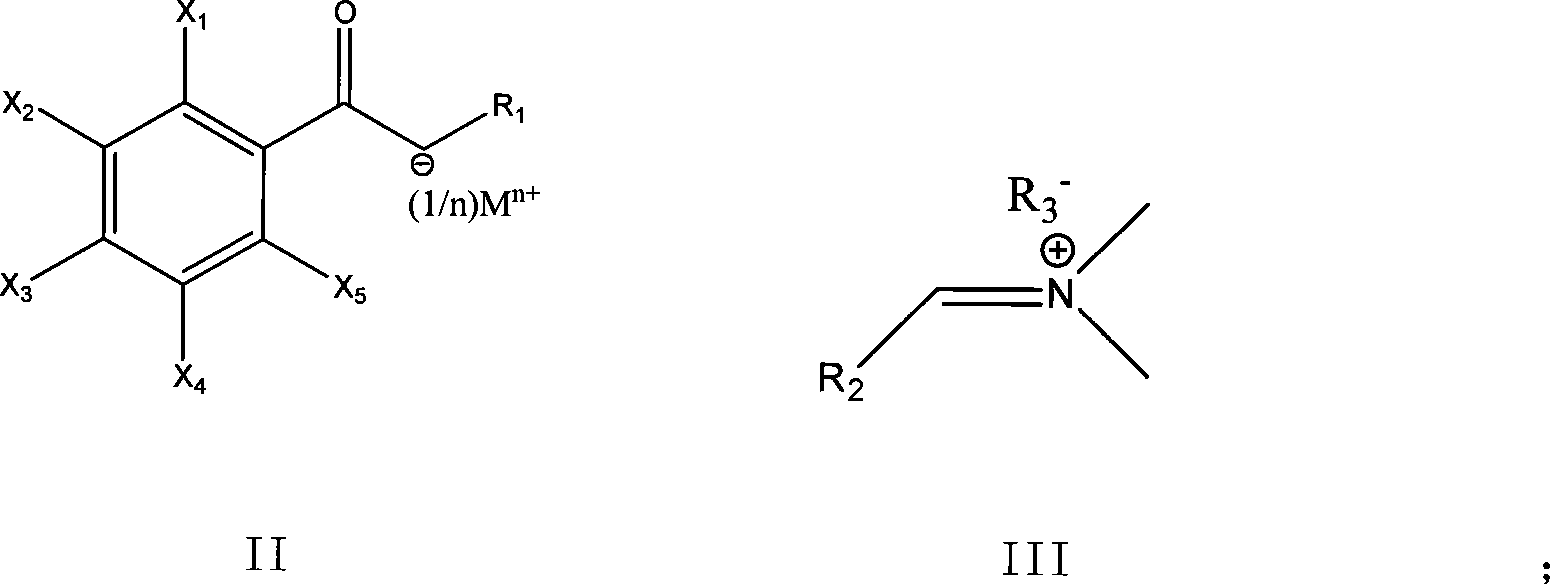
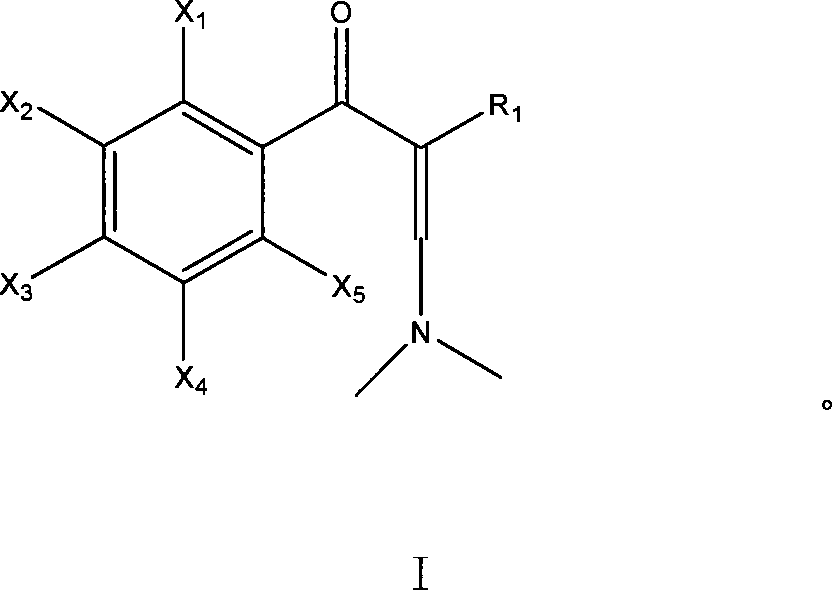
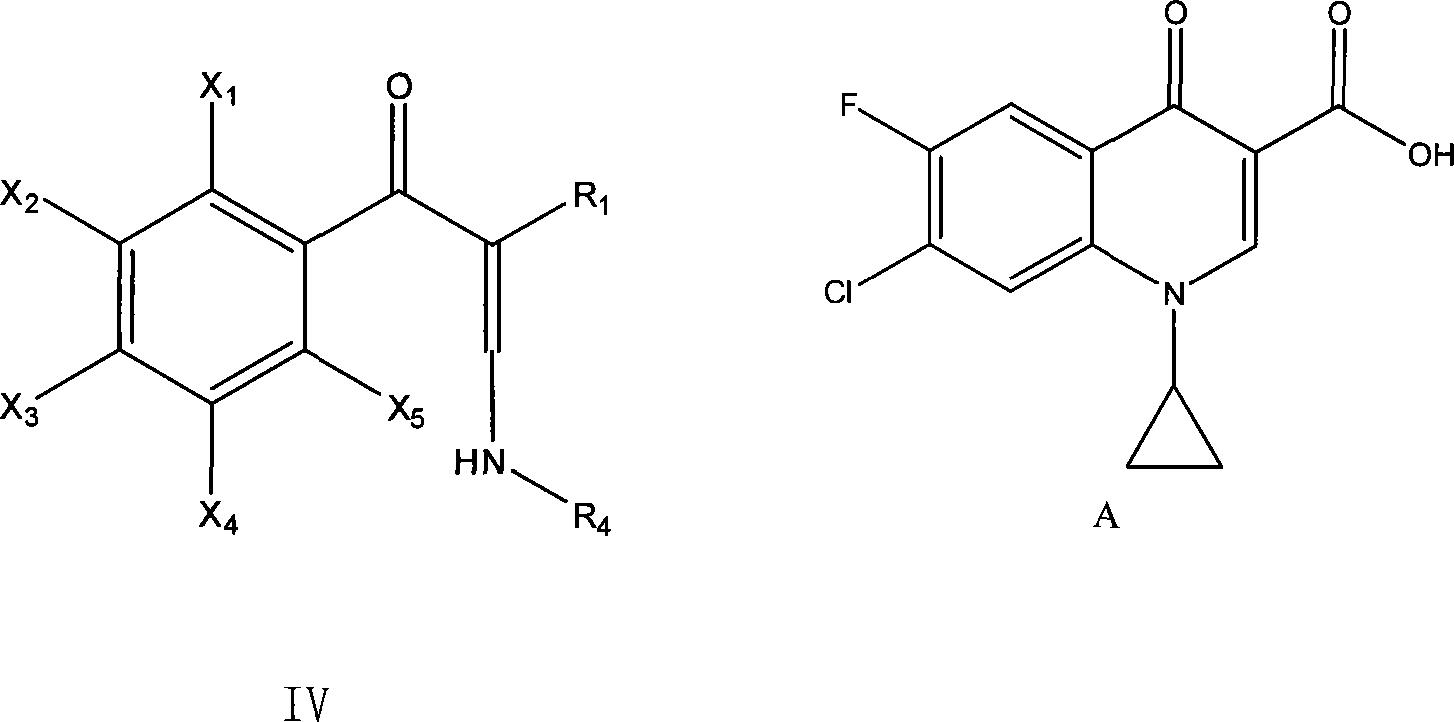
Method for preparing enamine derivates
A technology of derivatives and enamines, which is applied in the field of preparation of enamine derivatives, can solve the problems of large consumption of triethyl orthoformate and acetic anhydride, cumbersome preparation of DMFA, and unsuitability for large-scale production, and achieves low cost, high reaction The effect of mild conditions and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, the preparation of imide salt (Methoxymethylen) dimethylammonium-methylsulfat (molecular formula such as V):
[0049]
[0050] Add DMF (37.0g, 0.51mol) into a 250ml three-necked flask, stir and heat up to 55°C, slowly add dimethyl sulfate (63.1g, 0.50mol) dropwise, maintain the temperature in the flask at 50-60°C, and drop for about 60min complete. After dropping, raise the temperature to 75°C and keep it warm for 3 hours. After cooling, the imide salt can be obtained.
Embodiment 2
[0051]The preparation of embodiment 2, 3-(S-hydroxypropyl-2-amine)-2-(2,3,4,5-benzoyl) ethyl acrylate, the following steps are carried out successively:
[0052] 1. Throw 9.7g of sodium hydride and 200ml of toluene into a 500ml four-neck flask, and add dropwise 2,3,4,5-tetrafluorobenzoyl ethyl acetate solution (40g / 69ml of toluene, that is, 69ml Add 40g of ethyl 2,3,4,5-tetrafluorobenzoylacetate to toluene), drop it in about 2 to 3 hours, and react at 0°C for 8 hours to obtain 2,3,4,5- Toluene solution of ethyl tetrafluorobenzoyl acetate sodium salt.
[0053] 2. At about -5°C, add 61.5 g of the imide salt obtained in Example 1 dropwise to the toluene solution of the above-mentioned 2,3,4,5-tetrafluorobenzoyl ethyl acetate sodium salt (the molar ratio is 1: 2.04 ), the drop was completed in about 1.5 hours, and after the drop was completed, the reaction was incubated at -5°C for 15 hours, and the end point of the reaction was detected by spotting the plate. After the reaction...
Embodiment 3
[0055] Example 3, the preparation of 3-cyclopropylamino-2-(2,4-dichloro-5-fluorobenzoyl)acrylic acid acetonitrile, the following steps were carried out in sequence:
[0056] 1. Throw 6.5g of sodium hydride and 200ml of toluene into a 500ml four-neck flask. Control the temperature at about 0°C and add 2,4-dichloro-5-fluorobenzoyl acetonitrile solution (37g / 70ml toluene) dropwise, drop it in about 2 to 3 hours, and keep the reaction at the above temperature for 8 hours to obtain 2 , 4-Dichloro-5-fluorobenzoylacetonitrile sodium salt in toluene.
[0057] 2. Add dropwise 40 g of the imide salt (2,4-dichloro-5-fluoro The molar ratio of benzoyl acetonitrile to imide salt is 1:1.26), and the dripping is finished in about 2 hours; after the dripping, the temperature is kept at this temperature for 5 hours, and the temperature is raised to 40°C for 10 minutes after the warming, suction filtration, and the filtrate is recovered under reduced pressure To dryness, 3-dimethylamino-2-(2,4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com