Four kinds of tripterygium wilfordii derivative and preparing method of pharmaceutics thereof
A technology of triptolide and its derivatives, which can be applied in the direction of anti-inflammatory agents, drug combinations, and pharmaceutical formulations, and can solve problems such as hindering research and development, and large toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
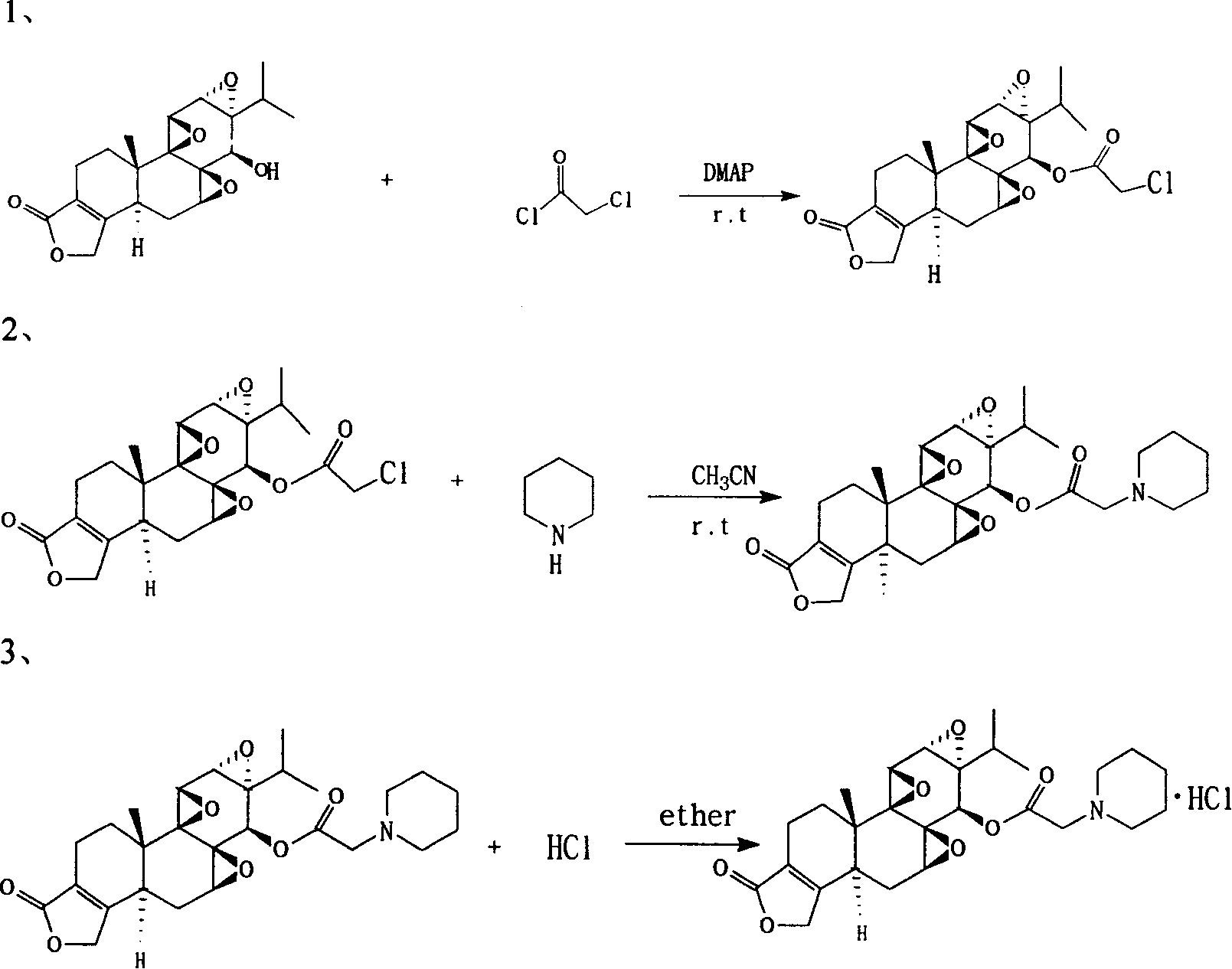
[0031] Synthesis of 2-(1-piperidinyl) triptolide acetate
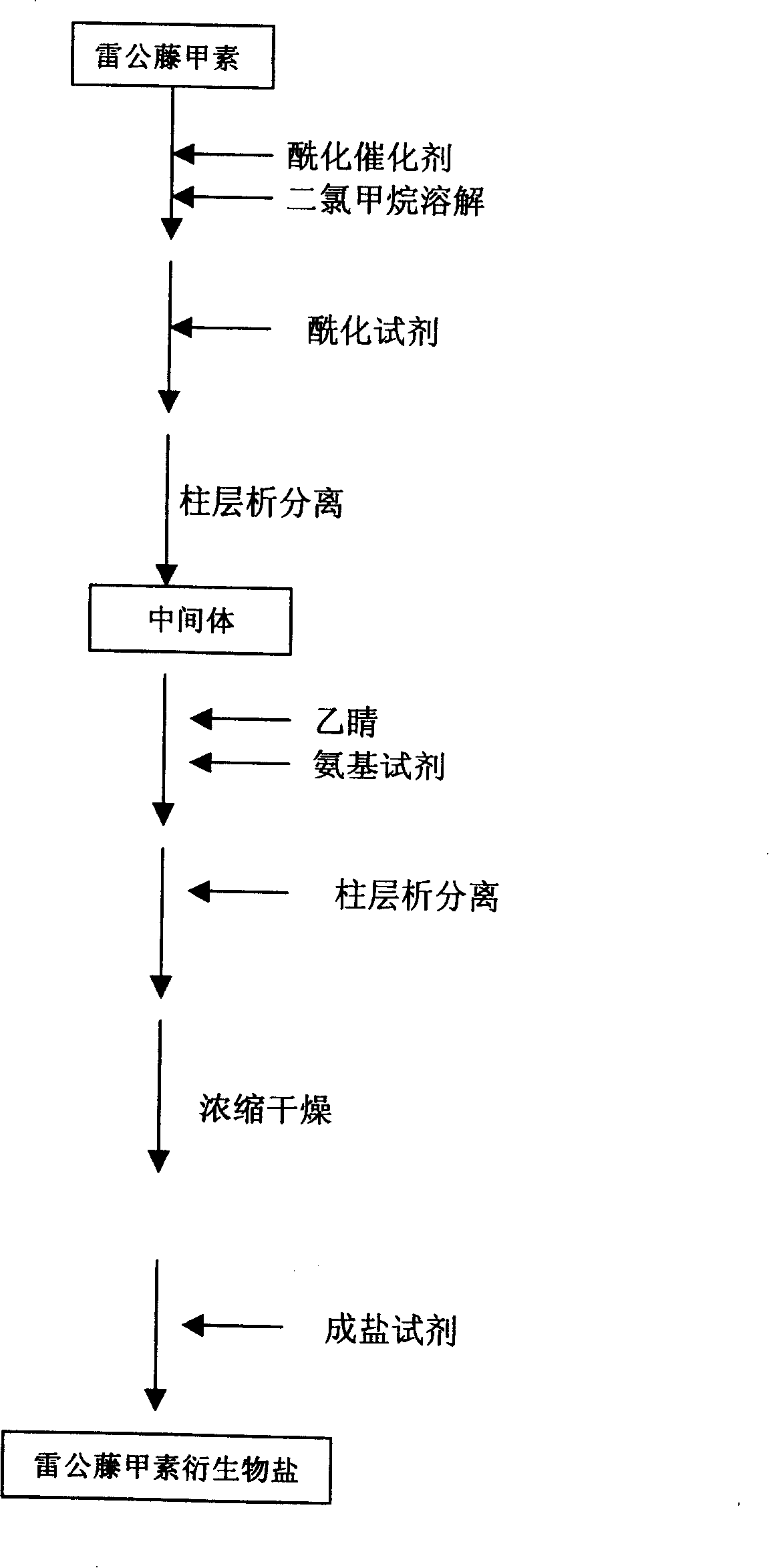
[0032] Put 1 gram of triptolide and 1.70 grams of dimethylaminopyridine in a 100-ml round-bottomed flask, add 35 ml of dichloromethane to dissolve, and add 0.78 ml of chloroacetyl chloride dropwise in an ice-water bath. Stir overnight at room temperature, and monitor the completion of the reaction by thin-layer chromatography on a silica gel plate, add 20 ml of 0.5 equivalent hydrogen chloride solution for washing, and then add ice water for washing. Collect the dichloromethane solution, add anhydrous sodium sulfate for dehydration, filter, and concentrate at room temperature to obtain 1.26 g of solid.
[0033] 1.26 g of the solid was dissolved in dichloromethane, separated and purified with a silica gel column, eluted with a mobile phase of cyclohexane:ethyl acetate (1:1), detected by thin-layer chromatography on a silica gel G plate, and the color-developed part was collected. It was concentrated under reduced press...
Embodiment 2
[0037] Preparation of triptolide hydrochloride 2-dimethylaminoacetate
[0038] Put 1 gram of triptolide and 1.70 grams of dimethylaminopyridine in a 100-ml round-bottomed flask, add 35 ml of dichloromethane to dissolve, and add 0.78 ml of chloroacetyl chloride dropwise in an ice-water bath. Stir overnight at room temperature, and monitor the completion of the reaction by thin-layer chromatography on a silica gel G plate, add 20 ml of 0.5 equivalent hydrogen chloride solution for washing, and then add ice water for washing. Collect the dichloromethane solution, add anhydrous sodium sulfate for dehydration, filter, and concentrate at room temperature to obtain 1.61 g of solid.
[0039] 1.26 g of the solid was dissolved in dichloromethane, separated and purified with a silica gel column, eluted with a mobile phase of cyclohexane:ethyl acetate (1:1), detected by thin-layer chromatography on a silica gel G plate, and collected the colored part. Concentrate under reduced pressure a...
Embodiment 3
[0044] Preparation of 2-Dimethylaminoacetate Triptolide Hydrochloride Lyophilized Powder for Injection
[0045] Weigh 1 g of 2-dimethylaminoacetate triptolide hydrochloride, 4.5 g of sodium chloride, add distilled water to 500 ml, stir to dissolve, filter with a 0.22 micron membrane filter, and pack into 5 ml vials. 1 ml per vial, lyophilized. Get 500 bottles of freeze-dried powder injections containing 2 mg of triptolide hydrochloride per bottle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com