Method for preparing bone morphogenic protein BMP-2 mature peptide
A morphogenetic protein, BMP-2 technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, peptide sources, etc., can solve the problems of immunogenicity, inconsistent amino acid sequence of BMP-2, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Removal of DsbA signal peptide gene
[0070] Primer design: DsbA-1: 5'AAGCATATGGCGCAGTATGAAGAT 3'24bp
[0071]DsbA-2: 5' TCGCTTAAGTATTTCACTGT 3' 20bp
[0072] PCR template: pET39 plasmid
[0073] PCR parameters: 94°C 3min
[0074] 94℃ 30s 40℃ 30s 72℃ 1min 3 cycles
[0075] 94℃ 30s 48℃ 30s 72℃ 1min 27 cycles
[0076] 72°C 10min
[0077] 4°C 10min
[0078] The PCR product and pET39 plasmid were digested with NdeI and BspTI to replace the original DsbA gene in pET39 with a DNA fragment without signal peptide gene, and the obtained recombinant plasmid was named pET39(SP-).
Embodiment 2
[0079] Example 2: Acquisition of the gene encoding "Linker-6His-Eksite-BMP-2"
[0080] First, design the DNA fragment encoding "Linker-6His-Eksite" according to the preferred codons of Escherichia coli, the sequence is as follows: ATA CTTAAG CGAGAAAAAAGGTTCTGGTTCTGGTCATCATCATCATCATGATGACGATGACAAA, where the underlined part is the BspTI recognition sequence. In order to splice the DNA fragment with the BMP-2 gene smoothly, the following four primers were designed and spliced by SOE technology.
[0081] Primer Linker-1:
[0082] 5'ATA CTTAAG CGAGAAAAAAGGTTCTGGTTCTGGTCATCATCATCATCAT 3'46bp
[0083] Primer Linker-2:
[0084] 5'TTGCTTATGCTTTGCTTGTTTGTCATCGTCATCATGATGATGATGATGATGACC 3'54bp
[0085] Primer BMP2-1:
[0086] 5'GACGATGACAAA CAAGCAAAGCATAAGCAA 3' 30bp
[0087] Primer BMP2-2:
[0088] 5'TTCGGATCCTTAGCGACAGCCACAACCTT 3' 30bp
[0089] Operation method:
[0090] Since primer Linker-1 and primer Linker-2 have 18bp reverse complementarity, take 2uL each of prime...
Embodiment 3
[0098] Embodiment 3: construction of engineering bacteria
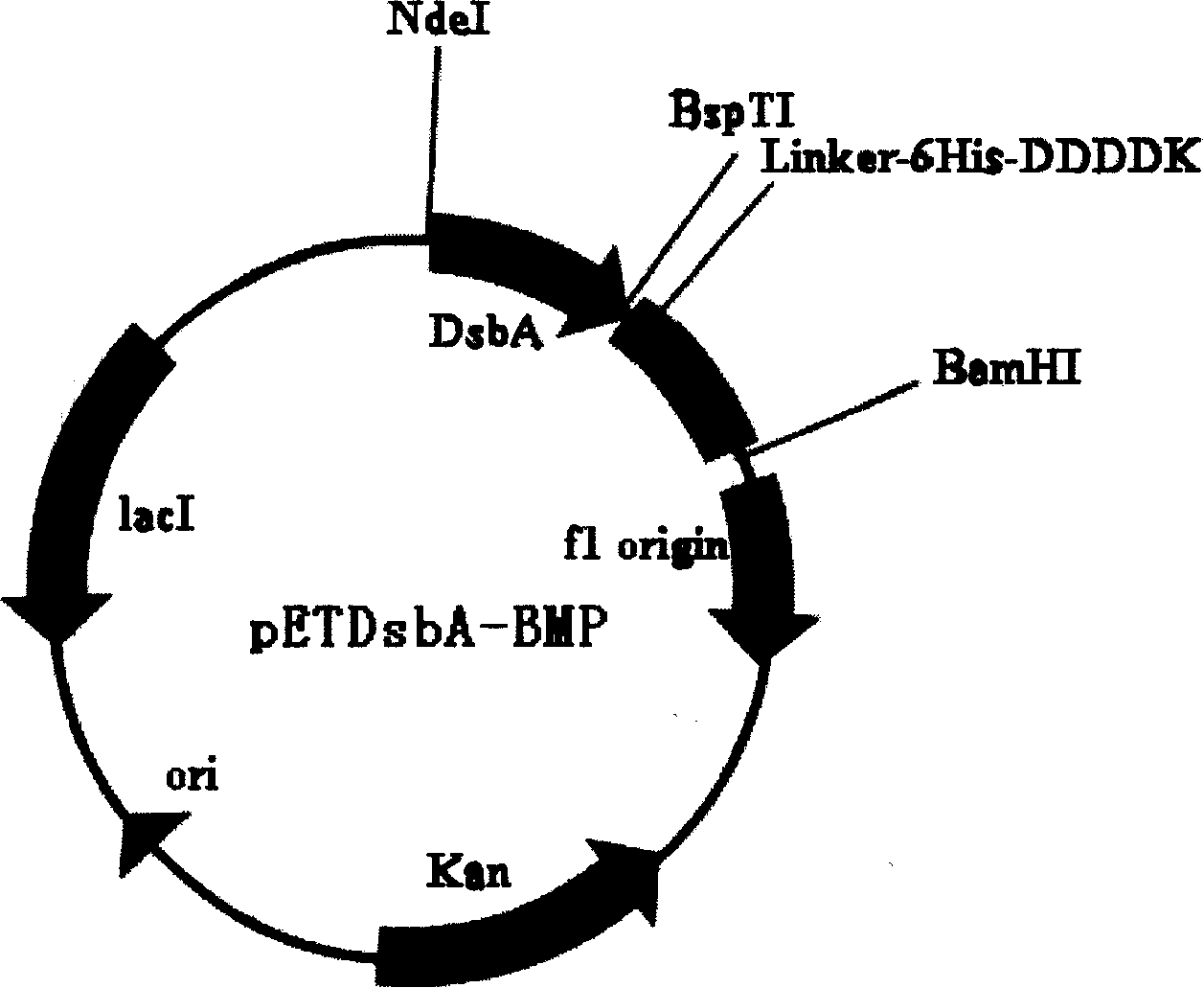
[0099] The product C was digested with BspTI and BamHI, and recombined into pET39(SP-) according to conventional molecular biology techniques to obtain the recombinant plasmid pETDsbA-BMP (see figure 1 ), the recombinant plasmid pETDsbA-BMP was transformed into E.coli BL21(DE3), and the engineered bacteria were preserved after sequencing and identification. For expression research and production.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com