Method for preparing iso-butyraldehyde and isobutyl alcohol by methylacrolein hydrogenation
A technology of methacrolein and isobutanol, which is applied to the preparation of carbon-based compounds, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve the problem of harsh reaction conditions, no reports of isobutyraldehyde or isobutanol, and poor catalyst stability and other problems, to achieve the effect of low raw material cost, high product selectivity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
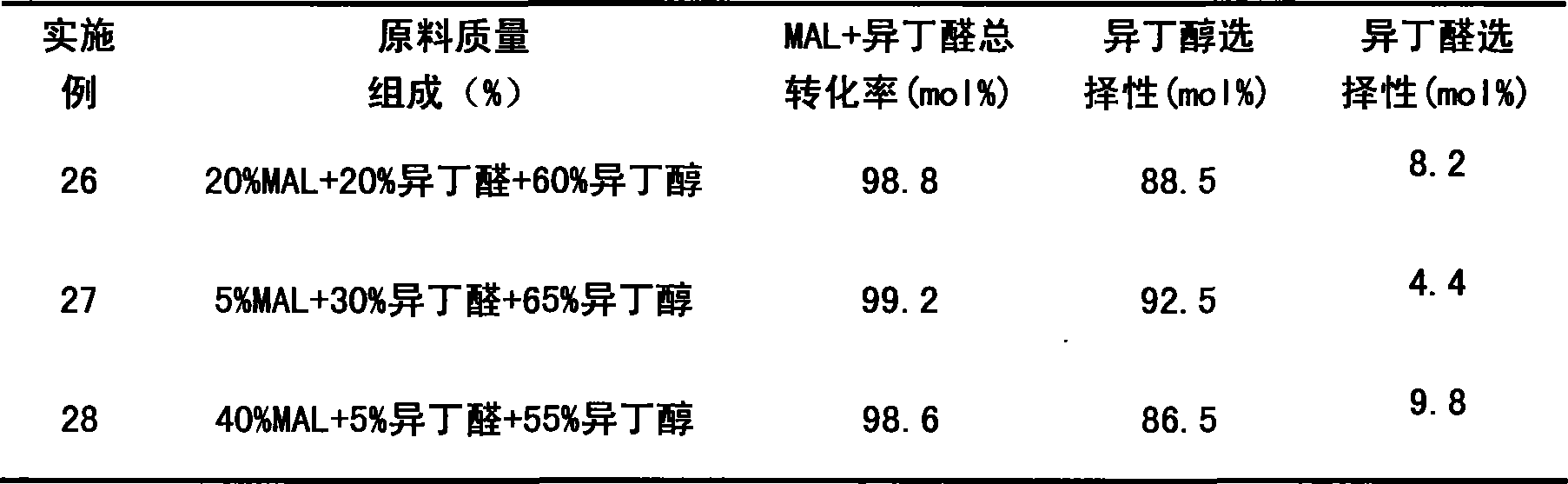
Examples
Embodiment 1~12
[0026] Catalyst preparation and activity test by impregnation method:
[0027]Immerse an appropriate amount of carrier of 20 to 40 meshes in the measured amount of soluble salt solution of catalyst active components and additives, evaporate to dryness in a water bath at 90°C, dry at 110°C for 6 hours, and roast at high temperature for 4 hours to prepare a catalyst precursor. Take 100g of into a fixed-bed continuous reaction device for reduction activation and catalyst performance evaluation.
[0028] The catalyst was treated with H before hydrogenation 2 Or hydrogen-containing mixed gas at 2.0MPa, H 2 Airspeed 500h -1 Conditional reduction treatment. After the temperature drops to the reaction temperature, the MAL solution of a certain concentration is fed into the preheater at a certain flow rate by a metering pump for preheating, and then enters the reactor to react with the hydrogen gas flowing through the catalyst bed at the same time, and the flow rate of the hydrogen ...
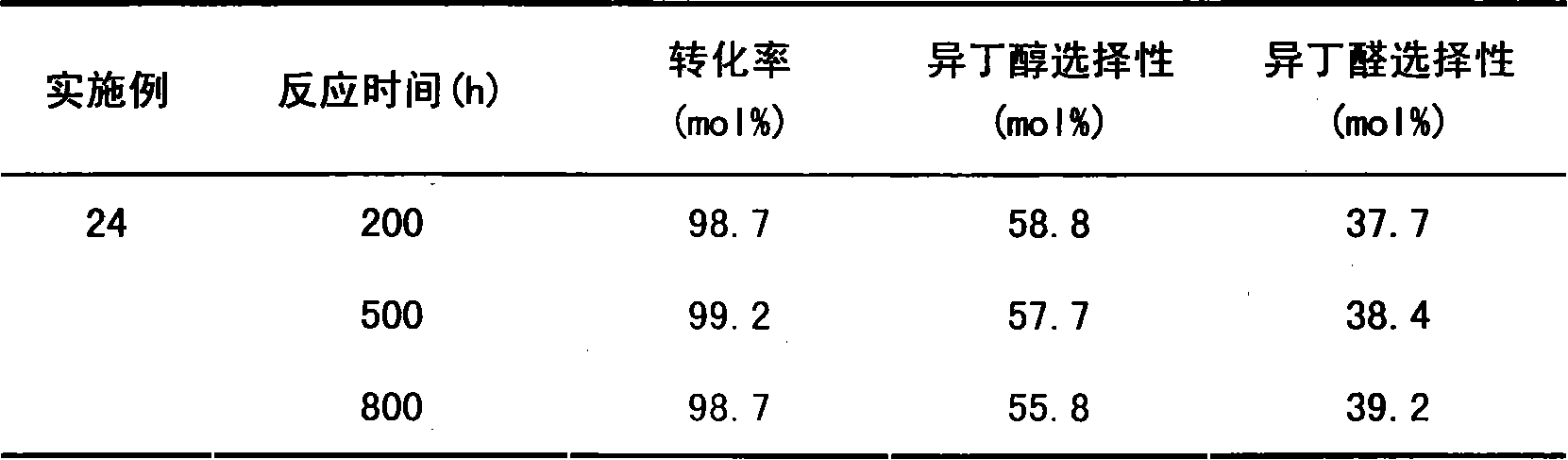
Embodiment 13~24
[0034] Catalyst preparation and activity test by co-precipitation method:
[0035] Prepare the mixed solution of the metered catalyst active component and the soluble salt of the auxiliary agent, and add the precipitant ammonia water, Na 2 CO 3 Or aqueous solution of alkaline substances such as NaOH, the resulting precipitate is aged at 75°C, then filtered, washed to neutral, dried at 90°C for 12 hours, and calcined at 550°C for 5 hours to obtain a catalyst precursor, shaped to 20-40 mesh Afterwards, 200 g was taken and loaded into a fixed-bed continuous reaction device for reduction activation and catalyst performance evaluation.
[0036] Catalyst reduction with 10% H 2 -90%N 2 The mixed gas was reduced for 4 hours under the conditions shown in Table 3. After the temperature drops to the reaction temperature, the MAL solution with a certain concentration is fed into the preheater with a metering pump at a certain flow rate for preheating, and then enters the reactor to re...
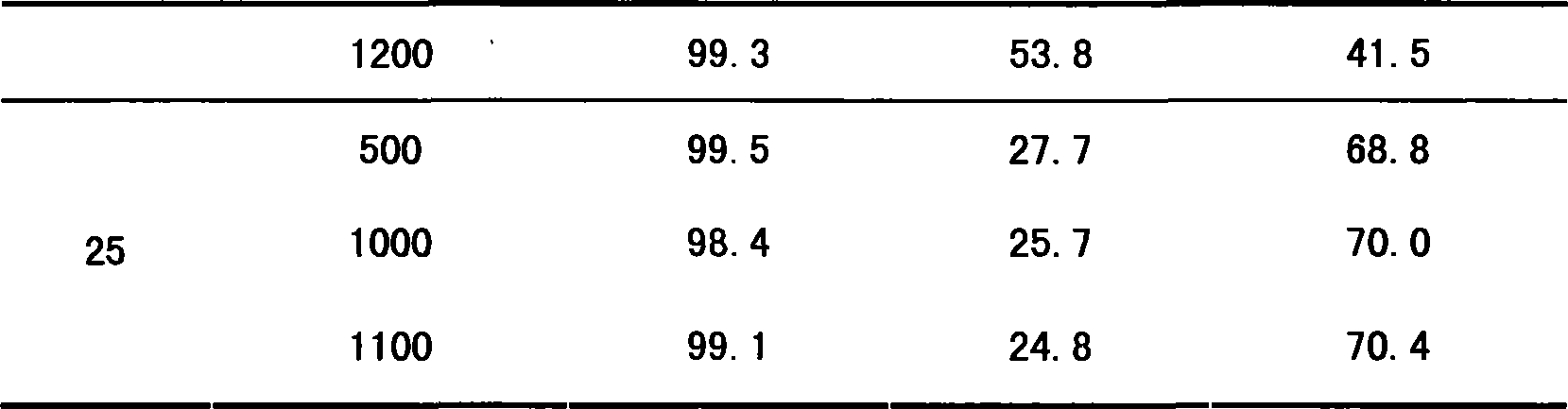
Embodiment 21~23
[0043] The catalyst in Example 12 was changed on the same reaction device as in Example 12 to change the reaction process conditions, and the reaction performance of the catalyst was investigated, as shown in Table 5. As can be seen from Table 5, the ratio of isobutanol and isobutyraldehyde in the product can be changed by adjusting the reaction process conditions, so that the product is mainly isobutyraldehyde or isobutanol.
[0044] The reaction condition and reaction result of table 5 embodiment 21~23
[0045] Reality
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com