Heterocyclic compound capable of inversing tumor cell drug tolerance, preparation method and application thereof
A technology for heterocyclic compounds and tumor cells, applied in the field of heterocyclic compounds and heterocyclic compounds that can reverse the drug resistance of tumor cells, can solve the loss of clinical efficacy, drug resistance, multi-drug resistance and cross-drug resistance of anti-tumor drugs, etc. problem, to achieve the effect of reducing the multiplier of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
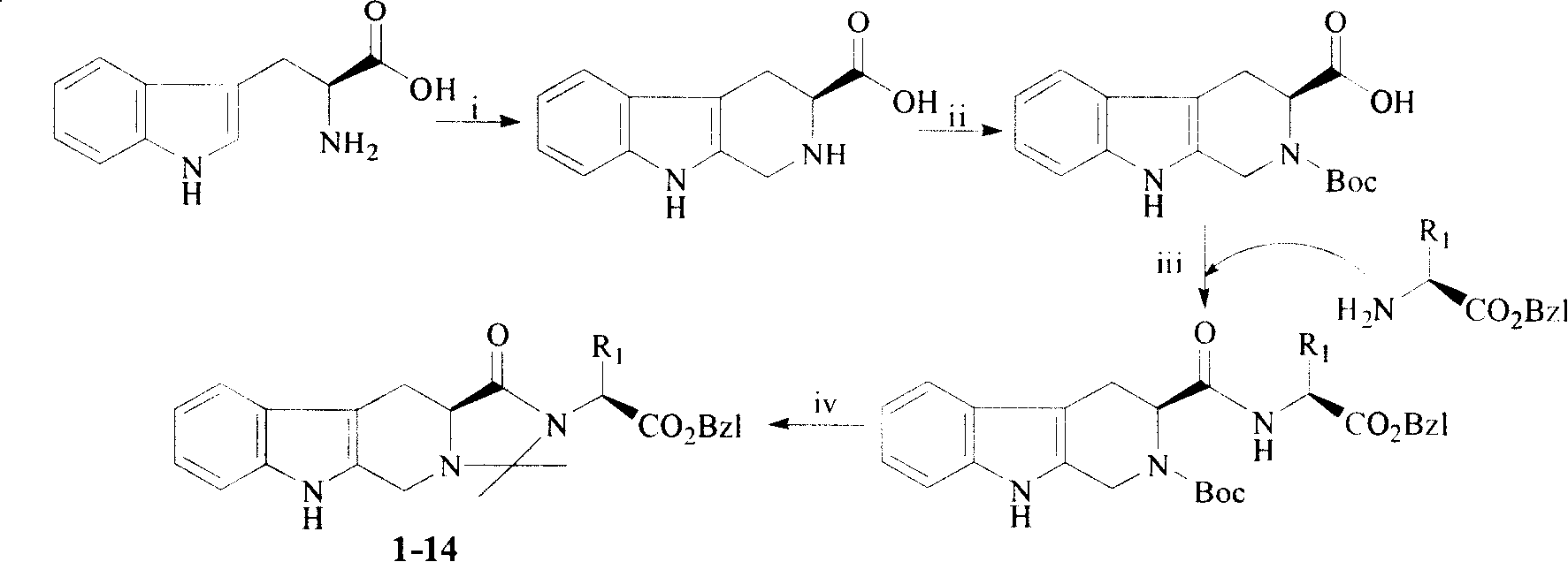
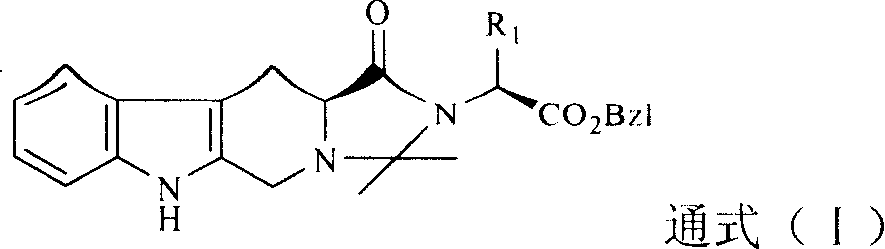
[0028] Example 1 1-(1'-Benzyloxycarbonyl-2'-methyl)butyl-2,2-dimethyl-4-oxo-tetrahydroimidazo[1',2':1,5] Preparation of pyrido[3,4-b]indole (compound 1)
[0029] 1) Preparation of S-carboline carboxylic acid
[0030] Put 400ml of water in a 500ml round bottom flask, and slowly add 0.2ml of concentrated sulfuric acid. Add 5.0 g (24.5 mmol) of L-tryptophan to the obtained dilute sulfuric acid aqueous solution and ultrasonically shake until the L-tryptophan is completely dissolved. To the resulting solution was added 10 ml of a 35% formaldehyde solution. The reaction mixture was stirred at room temperature for 6 hours. The disappearance of L-tryptophan was detected by thin layer chromatography, and the reaction was terminated. Concentrated ammonia water was slowly added dropwise to the reaction solution, the reaction mixture was adjusted to pH 6, and left to stand for half an hour. The resulting precipitate filtered out under reduced pressure was washed with water, and the fi...
Embodiment 2
[0037] Example 2 N-(1'-Benzyloxycarbonyl-2'-methyl)propyl-2,2-dimethyl-4-oxo-tetrahydroimidazo[1',2':1,5] Preparation of pyrido[3,4-b]indole (compound 2)
[0038] 1) Preparation of N-Boc-S-carbolinyl-L-valine benzyl ester
[0039] According to the operation of preparing N-Boc-S-carbolinyl-L-isoleucine benzyl ester in Example 1, 3.90 g (94%) of the target was obtained from 2.00 g (8.21 mmol) of L-valine benzyl hydrochloride Compound, a colorless solid. ESI / MS 506[M+H] + .
[0040] 2) N-(1'-Benzyloxycarbonyl-2'-methyl)propyl-2,2-dimethyl-4-oxo-tetrahydroimidazo[1',2':1,5]pyridine Preparation of a[3,4-b]indole (2)
[0041]According to Example 1, N-(1'-benzyloxycarbonyl-2'-methyl)butyl-2,2-dimethyl-4-oxo-tetrahydroimidazo[1',2':1, 5] The operation of pyrido[3,4-b]indole, 1.06g (60%) of the target compound was obtained from 2.00g (3.96mmol) N-Boc-S-carbolinyl-L-valine benzyl ester , a colorless solid. Mp 196-198°C; ESI + -MS(m / e)446[M+H] + ; 1 H-NMR (DMSO-d 6 , 300MHz) ...
Embodiment 3
[0042] Example 3 1-(1'-Benzyloxycarbonyl-3'-methyl)butyl-2,2-dimethyl-4-oxo-tetrahydroimidazo[1',2':1,5] Preparation of pyrido[3,4-b]indole (compound 3)
[0043] 1) The preparation of N-Boc-S-carboxyloyl-L-leucine benzyl ester according to the operation of Example 1 to prepare N-Boc-S-carborinyl-L-isoleucine benzyl ester, from 1.70g (6.61 mmol) L-leucine benzyl hydrochloride yielded 3.23 g (98%) of the title compound as a colorless solid. ESI + -MS(m / e)520[M+H] + ;
[0044] 2) 1-(1'-Benzyloxycarbonyl-3'-methyl)butyl-2,2-dimethyl-4-oxo-tetrahydroimidazo[1',2':1,5]pyridine Preparation of a[3,4-b]indole (3)
[0045] According to Example 1, N-(1'-benzyloxycarbonyl-2'-methyl)butyl-2,2-dimethyl-4-oxo-tetrahydroimidazo[1',2':1, 5] The operation of pyrido[3,4-b]indole, 0.97g (55%) of the target compound was obtained from 2.00g (3.85mmol) N-Boc-S-carbolinyl-L-leucine benzyl ester , a colorless solid. Mp 190-192°C; ESI + -MS(m / e)460[M+H] + ; 1 H-NMR (DMSO-d 6 , 300MHz) δ=10....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com