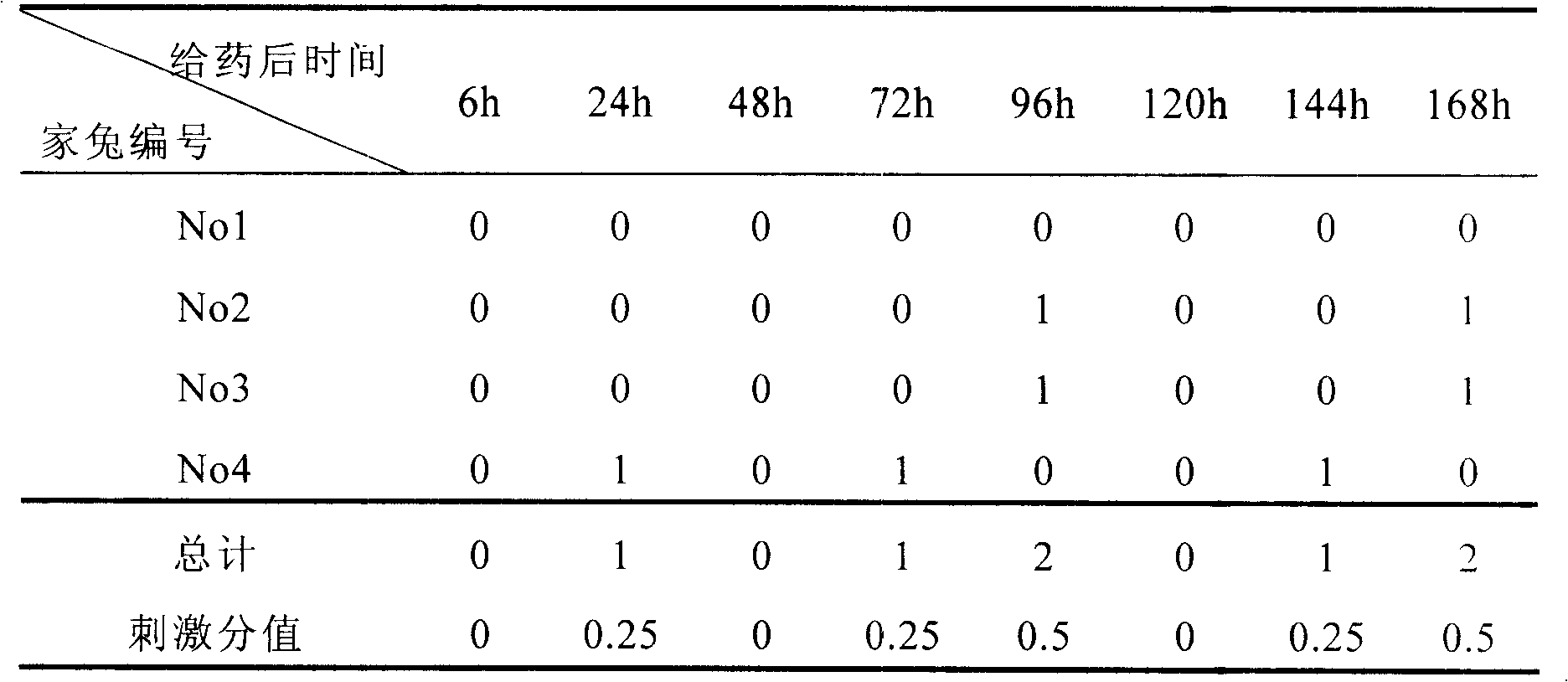
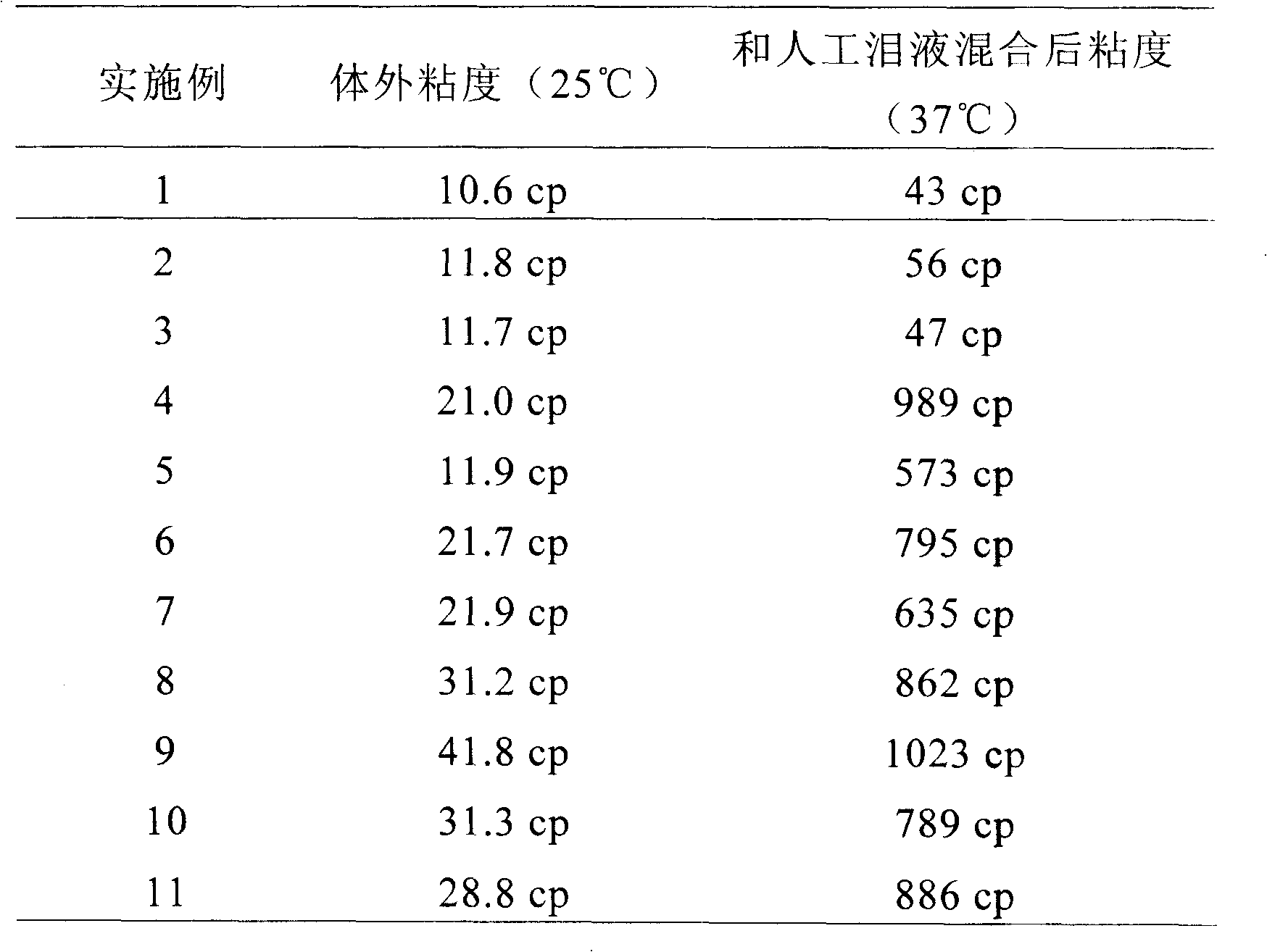
Micro/sub-micro emulsion in situ gel rubber preparation of cyclosporins A for eyes and preparation thereof
A technology of in-situ gel and cyclosporine, which is applied in the direction of medical preparations of non-active ingredients, cyclic peptide ingredients, pharmaceutical formulas, etc., can solve the inconvenience of industrial production and filling, does not have pseudoplastic deformation, and clinical Inconvenient medication and other problems, to achieve the effect of easy and accurate control of dosage, good toxic and side effects, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Prescription composition:
[0052] Cyclosporin A 0.5g
[0053] Castor oil 6g
[0054] Polyoxyethylene castor oil 15g
[0055] Sodium Alginate 10g
[0056] Glycerin 26g
[0057] Appropriate amount of borax
[0058] Benzalkonium Chloride 0.05g
[0059] Add purified water to 1000ml
[0060] Preparation method: weigh each component according to the prescription composition, mix castor oil, polyoxyethylene castor oil and glycerin (6g) uniformly at 60°C, and then dissolve cyclosporin A in the mixture. Add an appropriate amount of purified water, shear and emulsify to obtain colostrum, and then use a high-pressure homogenizer to homogenize (the pressure is about 140,000kPa, homogenize for 4 hours), and the obtained product is filtered with a 0.45μm microporous membrane; then the alginic acid Sodium and the remaining amount of glycerin (20g) were dissolved in an appropriate amount of purified water, and then mixed with the emulsion, adjusted to pH 6.2-8 with borax soluti...
Embodiment 2
[0066] Prescription composition:
[0067] Cyclosporin A 4g
[0068] Castor oil 6g
[0069] Tween 80 45g
[0070] Propylene glycol 30g
[0071] Gellan Gum 10g
[0072] Glycerin 2g
[0073] Appropriate amount of trishydroxymethyl aminomethane
[0074] Chlorobutanol 0.5g
[0075] Add purified water to 1000ml
[0076] Preparation method: weigh each component according to the composition of the prescription, mix castor oil, Tween 80 and propylene glycol uniformly at 60°C, and then dissolve cyclosporin A in the mixture. Add an appropriate amount of purified water, shear and emulsify to obtain colostrum (pressure about 60,000kPa, homogenize for 1 hour), and filter the obtained colostrum with a 0.45μm microporous membrane; then dissolve gellan gum and glycerin in an appropriate amount of purified water, and then mix it with the emulsion, and adjust the pH to 6.2-8.0 with tris hydroxymethylaminomethane solution; then, add the prescribed amount of chlorobutanol, and make up to 1...
Embodiment 3
[0078] Prescription composition:
[0079] Cyclosporin A 3g
[0080] Tricaprylic Capric Glyceride 6g
[0081] Polyoxyethylene hydrogenated castor oil 15g
[0082] Sodium Alginate 15g
[0083] Glycerin 20g
[0084] Appropriate amount of sodium hydroxide
[0085] Benzalkonium Bromide 0.05g
[0086] Add purified water to 1000ml
[0087] Preparation method: weigh each component according to the composition of the prescription, mix tricaprylic capric glyceride and polyoxyethylene hydrogenated castor oil uniformly at 60°C, and then dissolve cyclosporin A in the mixture. Add an appropriate amount of purified water, shear and emulsify to obtain colostrum, and then use a high-pressure homogenizer to homogenize (the pressure is about 100,000kPa, homogenize for 1 hour), and the obtained product is filtered with a 0.45μm microporous membrane; then the alginic acid Dissolve sodium and glycerin in an appropriate amount of purified water, mix it with the emulsion, and adjust the pH to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com